ATOMIC NUCLEUS: How Nuclei Change as a Result of Radioactive Decay, Mass Defect and Binding Energy #3
Dear esteemed readers. It’s been a pleasure writing on this platform (STEMsocial) once again. My post today is a sequel to one I wrote last, ATOMIC NUCLEUS. In that post, I explained in details, the binding energy curve and the six main ways in which unstable nuclei decay or change. In this present post, I’ll like to explain how a nuclear disintegration may cause a chain reaction in which more nuclei split as they absorb neutrons, the critical mass, and structure, uses and troubles of nuclear reactors.
NUCLEAR DISINTEGRATION CAUSING CHAIN REACTION
The binding energy curve per nucleon shows that for nuclei larger than the iron nucleus at the low point of the curve, energy might be generated by splitting a nucleus into two parts. For this to work, both new nuclei must be near the bottom of the curve. The best-known fissionable nucleus is of uranium-235. The nucleus can split spontaneously, a very rare event, but one that can be triggered by the nucleus absorbing a neutron.
A simple but workable model for this is to imagine a large nucleus to be a drop of liquid, with the net nuclear-attracting force acting like the surface tension that keeps a water drop together. A neutron entering this nucleus will set it oscillating like a disturbed drop of mercury in a dish, say. If the nucleus forms a dumb-bell shape it may split into two parts. Like the liquid drops, two smaller nuclei have less energy than the single drop or nucleus. Both new nuclei are nearer the bottom of the nuclear valley described earlier.
The fission (disintegration) products, barium and krypton, are just two of many possibilities. Note that two neutrons are also released. This means that a nuclear disintegration may cause a chain reaction in which more nuclei split as they absorb the released neutrons.
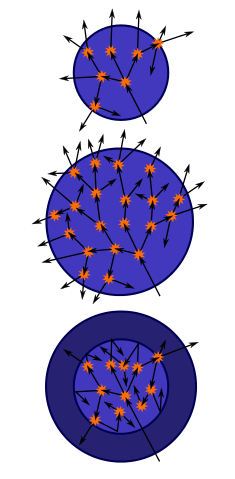
CRITICAL MASS
In a small mass of fissile material – such as uranium enriched with uranium-235 – some natural (spontaneous) fission occurs all the time, producing neutrons. Most of them escape from the mass completely, and so do not cause fission of other nuclei. This is because any lump of matter is mostly empty space, and the chance of a neutron colliding with the tiny nucleus of an atom is very small.
But suppose the mass, and so the size of the fissile material, is increased. There are more neutrons released and there is also an increase in the total ‘target area’ of nuclei for each neutron. So there will be more induced fission, and more energy will be released in the mass – it will get hotter.
Explosion or meltdown?
This is a classic case of positive feedback: the more fission, the more neutrons are released, so there is even more fission. In a large enough mass of the fissile material, induced fission produces a runaway chain reaction: a very large number of nuclei will disintegrate in a very short time. There is a sudden very large release of energy – an explosion. For a given composition of material, the mass at which this will occur depends on its shape. But for a sphere, it is called the critical mass.
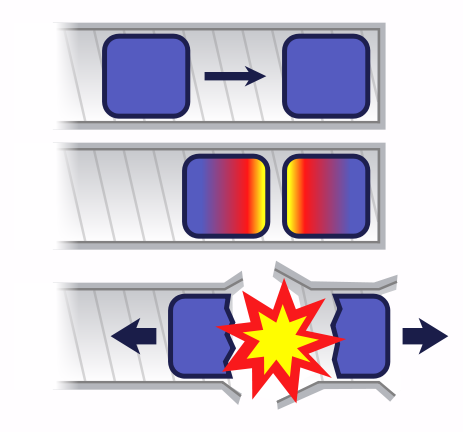
In practice, simply putting lumps of enriched uranium in a container will not have this effect. Before the critical mass is reached, the energy generated will melt (indeed, vaporize) the uranium. This is a ‘meltdown’, as happened in the worst nuclear reactor accident of all time at Chernobyl in 1986.
STRUCTURE OF A NUCLEAR REACTOR
In a nuclear reactor, energy is generated in fuel rods contained in thin non-corrosive tubes inserted into the reactor core. Around the core is a gas or water under pressure. The fuel rods contain either plutonium or uranium oxide, made with an ‘enriched’ mixture of 97 percent uranium-238 and 3 percent uranium-235. The core of a typical reactor contains about 80 tonnes of uranium oxide. Only the uranium-235 nuclei are likely to undergo fission, the ultimate energy source in the fuel rod.
Increasing induced fissions by using slow neutrons
The neutrons from spontaneous random fission of U-235 are emitted at high speeds. Fast neutrons have a much smaller probability of entering a nucleus than slow ones; they tend to bounce off. To slow down the neutrons, the fuel rods are surrounded by a moderator. This used to be graphite, but modern pressurized water reactors use the water which also acts as a coolant. The fast neutrons collide with coolant molecules and gradually lose kinetic energy until they are slow enough to have a much greater chance of causing induced fission when they reach the next fuel rod.
Controlling the reactions
Once induced fission starts, too many neutrons could be produced and the reaction could start ‘running away’. Therefore, excess neutrons must be removed, so that the fission rate is controlled and energy is released at a steady rate. To do this, cylindrical control rods are inserted into the reactor core near the fuel rods. The material of the control rods, for example, boron or cadmium, absorbs neutrons.

USING THE ENERGY FROM A NUCLEAR REACTOR
The fission products are large nuclei which separate at high speed. These collide with other nuclei and their kinetic energy is shared out, so that the reactor core containing the fuel rods, moderator and control rods becomes very hot. Nowadays, the reactor core contains and is surrounded by a fluid, most commonly water under pressure, as in pressurized water reactors (PWRS).
The energy is carried from the core by superheated water at a temperature of 325 °C kept from vaporizing by a pressure of 150 atmospheres. This water heats a secondary loop of water in a ‘heat exchanger’, and produces steam to drive a turbine that produces electricity.
The first and so far only, pressurized water reactor (PWR) in Britain is Sizewell B in Suffolk, which came on stream in 1995. Worldwide, it is one of the most popular types being built today. PWRs have a compact core because water is the most effective moderator for slowing down the neutrons. This means that the cores can be prefabricated in a factory and transported to the site. Any new reactors built in the UK are likely to be improved versions of the PWR which need less fuel, have little need for mechanical pumps, which might break down, and are easier to maintain. In case of an accident, reactor safety relies on passive features such as gravity and convection.
THE TROUBLE WITH NUCLEAR POWER
It seems that nuclear power avoids many of the problems we associate with most common energy source, fossil fuel. Nuclear power does not add greenhouse effect, which is mainly due to carbon dioxide from burnt fuel and its raw material will not run out as quickly as, say oil reserves. Nor does it produce the gases from burning coal and oil which affect health and cause acid rain.
But nuclear power has its own problems. There is the risk of accidents in which radioactive materials can escape into the environment. Also, even for perfectly managed systems, there are the problems of nuclear waste and of closing down (decommissioning) power stations at the end of their useful life. These are problems because they may expose people (and other forms of life) to harmful ionizing radiations as described below.
Nuclear waste
Nuclei of the non-radioactive material in the core may become radioactive isotopes when they absorb neutrons. Also, the spent fuel rods contain the fission products which tend to be radioactive, as well as the unused uranium-238. The uranium can be recycled, but eventually the main components of the reactor core become radioactive waste and have to be taken out.
Radioactive waste includes both short-lived isotopes which are highly active, and isotopes with very long half-lives (such as plutonium-239, half-life 24 000 years, formed from uranium-238). The waste is kept in storage tanks for years to allow the ‘high-level’ wastes to decay to less active or stable isotopes.
Nuclear fusion on Earth – the tokamak
The hydrogen bomb
Small nuclei can combine to make larger nuclei with the release of energy, as explained in the main article. This happens naturally in the extreme conditions in the centres of stars. On Earth, nuclear fusion was achieved artificially in the ‘hydrogen bomb’, first exploded in 1952. This used the uncontrolled chain reaction of nuclear fission (a fission bomb) to create a high enough temperature for isotopes of hydrogen (normal hydrogen and deuterium, 21H) to fuse together to form helium and release enormous energy. This is called a thermonuclear process. Such bombs are many thousands of times more destructive than the first nuclear bombs used on Japan in 1945.
Controlled fusion – the ultimate energy source?
Russia, the United States and the European Union have for many years tried to develop a working version of a controlled thermonuclear energy source. The main problems are to produce and maintain a high enough temperature for the process to begin, whilst at the same time keeping the very hot particles close enough together for fusion to continue, and in a controlled way.
The working gases are a mixture of two hydrogen isotopes, deuterium, and tritium (21H and 31H). At high temperature, the gas molecules separate into atoms and then into electrons and nuclei, so forming a plasma. The plasma is heated to a temperature of over 107 K to trigger a fusion reaction. Then, the nuclei have enough kinetic energy on average for a significant fraction of them to fuse on collision.
21H + 21H → 42He + 10n plus 17.59 MeV
Induction heating brings the gas to high temperatures: electric coils round the chamber act as the primary coils of a transformer and the plasma nuclei act as the secondary. Alternating current at high frequency in the primary coils induces a current of ions, which means the ions gain kinetic energy. The high temperature can also be reached by firing powerful lasers into the gas.
At these high temperatures, the ordinary solid matter of a container would melt or evaporate. So the plasma has to be held in a ‘magnetic bottle’: a strong field makes the high-speed nuclei move in circles or tight spirals in the shape of a hollow doughnut – a torus – well away from the walls of the chamber.

In 1997 the Joint European Torus (JET) tokamak produced 16 MW of power for 2 seconds – a world record. Even so, more energy was supplied to the machine to produce the result than was obtained from fusion.
The advantages of fusion as a source of energy are that the raw material is deuterium, which is easily extracted from the sea, and the end product is non-radioactive helium. However, highly radioactive tritium is formed during the reaction. Also, neutrons released during fusion are captured by the chamber walls, so making new, radioactive isotopes. It is therefore unlikely that controlled fusion will become a practical energy source until well into the twenty-first century.
A fusion plant could operate for a whole year, generating 7 billion kilowatt-hours of electricity, using just 100 Kg deuterium and 3 tonnes of lithium. A coal-fired power station would generate 11 million tonnes of carbon dioxide to produce the same annual output.
In 2005 a group of countries containing half the world’s population agreed to build a new tokamak – the International Thermonuclear Experimental Reactor (ITER) – at Cadarache in France. This was budgeted to cost 5-10 billion euros and should be operational by 2035.source
Nuclear power station operators would like to store all wastes deep underground but it is difficult to get agreement on where to do this. The other problem is how to decommission a nuclear power station when it becomes obsolete and is no longer economical to run. A large, slightly radioactive mass of building material has to be made safe- and this could involve burying the whole site in concrete.
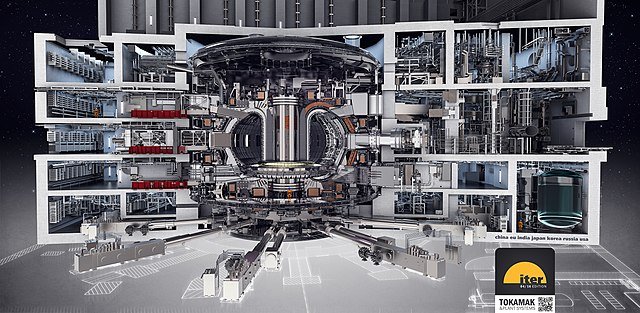
Drawing of the ITER tokamak and integrated plant systems. Oak Ridge National Laboratory, CC BY 2.0
Dealing with waste is so costly that nuclear power may be no cheaper than power from conventional power stations using fossil fuels, But, of course, the environmental cost of using fossil fuels is not yet paid. For example, the consequences of acid rain and the more distant and unknown effects of global warming are already be seen.
REFERENCES
http://www.atomicarchive.com/Fission/Fission2.shtml
https://www.britannica.com/science/nuclear-chain-reaction
https://en.wikipedia.org/wiki/Critical_mass
https://www.investopedia.com/terms/c/critical-mass.asp
https://www.britannica.com/technology/nuclear-reactor
https://www.energy.gov/ne/articles/nuclear-101-how-does-nuclear-reactor-work
https://www.eia.gov/energyexplained/nuclear/nuclear-power-and-the-environment.php
https://www.conserve-energy-future.com/nuclearenergy.php
https://en.wikipedia.org/wiki/Radioactive_waste
https://whatisnuclear.com/waste.html
https://en.wikipedia.org/wiki/Tokamak
https://time.com/4954082/hydrogen-bomb-atomic-bomb/
https://www.livescience.com/53280-hydrogen-bomb-vs-atomic-bomb.html
https://en.wikipedia.org/wiki/Thermonuclear_weapon
https://en.wikipedia.org/wiki/Fusion_power
https://link.springer.com/chapter/10.1007/978-1-4684-2607-6_7
https://www.vox.com/2014/4/16/5580192/the-comprehensive-guide-to-fusion-power
https://en.wikipedia.org/wiki/Joint_European_Torus
https://en.wikipedia.org/wiki/ITER
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Thanks for using the STEMsocial app, which gives you stronger support. Including @steemstem as a beneficiary could yield even more support next time.