Why is sodium chloride edible if it contains sodium and chlorine?
Sodium chloride, or common salt, has been used for thousands of years to seasoning and preserve foods, in addition to being used in other applications, so it has a long history of safe and regular use, being perhaps one of the few compounds more familiar chemicals, however, it is composed of two chemical elements with a different history, on the one hand there is chlorine, a chemical element so aggressive that it has even been used as a weapon, and on the other there is sodium, a highly reactive metal It reacts violently with water.

Elements that make up common salt. Source: @emiliomoron, images of sodium, chlorine and salt are the public domain.
So unless too much salt is ingested, it doesn't pose much of a threat to our health, but if we break this compound down into its constituent elements we could unleash terrifying chemical weapons. So, have you ever wondered why sodium chloride is harmless when its component atoms are so reactive and dangerous?
Let's see why this is
Chemical nature
As we very well know, all matter is made up of atoms, and its structure is what justifies its properties, so the number of protons in its nucleus determines the chemical identity of the atom, and the number of electrons, especially the valence electrons, are responsible for the chemical behavior of atoms.
So we must start by keeping in mind that neutral atoms and their ions have very different chemical behavior, even if they are the same atom.
Going back to the example, on the one hand we have that sodium atoms form metallic sodium, a soft, silvery alkaline metal that burns vigorously in air because it oxidizes easily in the presence of oxygen, and also reacts explosively with Water.

Sodium reaction in water. Source: Wikimedia.org.
And on the other hand, chlorine is an element belonging to the halogens, and under normal conditions its atoms form chlorine gas (Cl2), a toxic gas of yellow-green color, it is extremely corrosive to most metals and highly poisonous.

Chlorine gas in a bottle. Source: Wikimedia.org.
But, the fact is that table salt is composed of sodium cations (Na+) and chlorine anions (Cl-), and the compound formed by these ions has properties chemical very different from the elements from which the ions come, since they have a different atomic structure.
Specifically, it is the number of electrons in the valence shell that differentiates the atoms, sodium is a highly reactive element, in contact with water it easily gives up its valence electron, likewise chlorine is another very reactive element, being even up in the group of halogens means that it has a high electronegativity, which means that it tends to take away electrons from another atom, that is why sodium and chlorine chemically bond with great ease, for one it is easy to give up electrons and for the another accept them.
Let's see the above like this, the elements are rarely found in their elemental form in nature, this is because they are not stable forms, sodium for example has atomic number 11, which means that it has 11 protons in its nucleus and 11 electrons around it, so it acquires the electronic configuration [Ne] 3s1, and for this reason it is easier for it to give up that single electron in its valence shell to stay with a more stable structure such as Na+ ion , remaining with a complete external electronic configuration similar to that of neon, and like the noble gases, whose electronic configuration is complete, it tends not to react being that way.
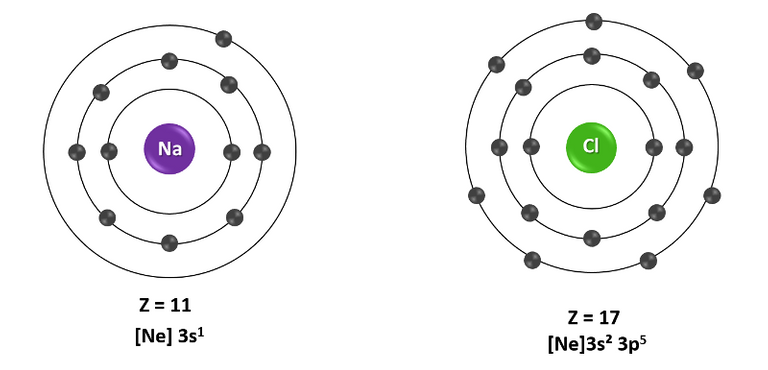
Electron shell diagram of neutral sodium and chlorine atoms. Source: image made in powerpoint.
For its part, chlorine has atomic number 17, which means that it has 17 protons in its nucleus and 17 electrons orbiting it, which makes its electronic configuration [Ne] 3s23p 5, so it ruthlessly seeks one more electron to acquire a complete outer shell (8 electrons), so by becoming an anion (Cl-) it acquires a complete outer shell isoelectronic with argon , this form being more stable. So this element is highly reactive towards elements that can easily give up an electron, like sodium.
So, these two atoms when they are close to each other have a high potential to bond, in this case, if a metal and a non-metal bond through the transfer of an electron, which is defined as an ionic bond, both atoms reach more stable electron configurations, and as a result, a less reactive product. This type of compound made up of anions and cations are usually crystalline in nature, such as salt.
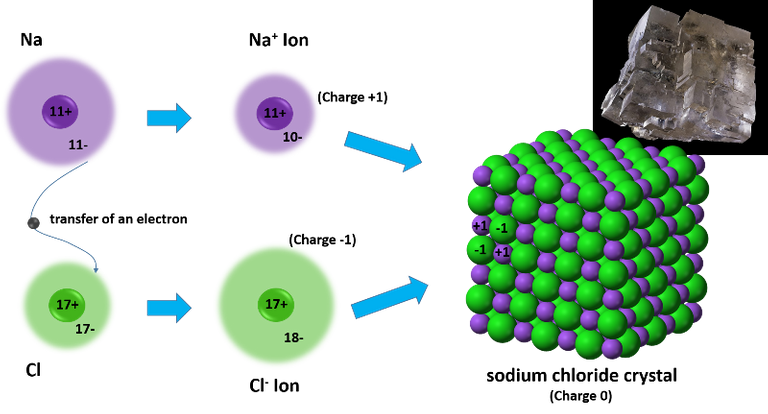
Reaction between sodium and chlorine atoms. Source: image made in powerpoint, the crystal structure of NaCl is from Wikipedia.com.
And why is this solid safe to eat?
When this compound made up of ions when in contact with water dissociates, the chemical way of saying that its ions separate from each other, forming free cations and anions in the solution, and because these cations and anions have a configuration stable electronics are less reactive than the neutral atoms from which they come.
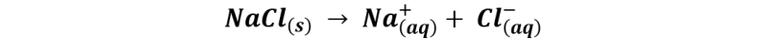
And the same thing happens when salt crystals are added to food or put in our mouths, they immediately dissociate into their ions, which are stable and less reactive forms than the dangerous neutral sodium and chlorine atoms.
Well friends, I hope you have liked the information on the chemical behavior of atoms and their ions. Until next time!
References
Atkins, P., Jones, L. (2006). Chemistry principles. 3rd edition, Médica Panamerica, Buenos Aires.
Wikipedia.com. Sodium
Wikipedia.com. Chlorine
Great Post!
!1UP
You have received a 1UP from @luizeba!
@stem-curator, @neoxag-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
This was a very clear post. Thanks a lot for sharing it with us.
As a minor comment, it may be good to define the spectroscopic notation for the electron configurations in the atoms, that most readers on the chain may not be familiar with.
Cheers!