What is a sacrificial anode and how does it work? Explained with an experiment
As we know, the issue of steel corrosion is very important to us, since it is basically one of the main construction materials, if not the most important one; it is enough to mention that it is the element that provides support to our buildings, with it our means of transport and most of the tools we use are built. So, it is perfectly understandable that at the same time that the consumption of iron for our structures is increasing, we have also developed methods to protect it from corrosion, which as we know, is a natural and almost inevitable process in most metals.
The usual way to protect iron from corrosion is through a layer of anti-corrosion paint, which provides a layer that isolates the metal from oxygen and moisture in the environment. However, in some cases this protection is not sufficient, especially in marine environments where the attack of corrosion is more severe. For these cases we use what is known as cathodic protection, which uses as a principle the difference in reduction potentials that have two metals that are in contact, perhaps we have noticed that not all metals oxidize as easily as iron, for example copper and aluminum are more resistant to corrosion. Then, using this chemical property, we can install an element of a less noble metal than iron in the steel structures we want to protect, so it will be this one that will oxidize instead of the iron of the structure. This is why these elements are known as "sacrificial anodes", since they will be the ones that will suffer corrosion and eventual deterioration.
Demonstration of cathodic protection with a zinc sacrificial anode. Source: @emiliomoron.
In this post I intend to explain how the sacrificial anode works, by performing an experiment that we can do with materials we have available at home.
Let's define some concepts
We must be clear that oxidation is a chemical process in which a species suffers loss of electrons[1], so, in a way, all substances oxidize; the difference is the speed with which they do so. When corrosion occurs, the oxidation-reduction reactions of an electrochemical cell are established, where some areas of the metal surface become the anode (where oxidation occurs) and other areas become the cathode (where reduction occurs).
A sacrificial anode is a very electropositive metal that has a tendency for high oxidation, basically it is a more active metal (with less potential) than the metal that forms the structure we want to protect, giving rise to what we call cathodic protection, because, being in contact with both metals and immersed in an electrolyte, the whole structure would act as the cathode of an electrolytic cell and the most active metal as the anode[2]. For example, zinc anodes are used to protect the hull of ships, as illustrated in the image below.

Zinc anode installed on a ship, new (up) and used (down). Source: Wikipedia.com, image CC BY-SA 3.0
As we can see, the one that is suffering from corrosion is the zinc element installed in the ship, so from time to time it will have to be replaced.
Choosing the right metal
The use of a metal as a sacrificial anode will depend on the metal of the structure to be protected and the characteristics of the electrolyte in which they will be in contact. But in general, for cathodic protection to work, the metal that will function as a sacrificial anode must have a lower reduction potential than the potential of the metal that makes up the structure to be protected, taking a copper/copper sulphate electrode as a reference. In the following image we can see the potential of some metals.
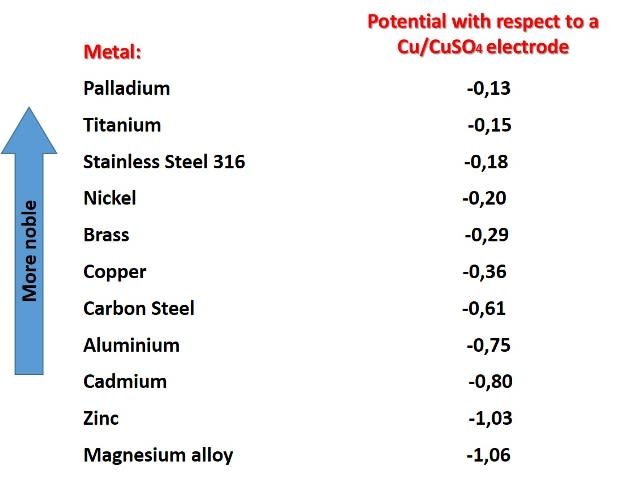
Reduction potentials of some metals. Source: Author's image with data taken from Wikipedia.com
According to the image not any metal can provide protection to steel, only those below it on the list, such as aluminum, zinc or magnesium. And that in fact, are the three metals mainly used as sacrificial anodes.
Testing the theory
To start we need the following materials:
- A pair of normal iron nails
- A piece of zinc sheet
- A piece of copper wire
- And a disposable plastic plate
- Water with a little dissolved salt

Metals to be used in the experiment. Source: @emiliomoron.
The procedure is very simple, we will take one of the nails and in one end we will roll up the piece of copper wire, in a similar way, we will take the other one and we will roll up the piece of zinc sheet (this metal can be obtained from the metallic cover of a zinc-carbon pile).

Then we place both nails in the plastic plate and add enough water with dissolved salt to cover half of the nails and leave part of their surface exposed. Since corrosion is an electrochemical process, salt provides the appropriate electrolytic medium to accelerate the process.

Nails placed in the solution. Source: @emiliomoron.
After a few hours we will begin to observe that around the nail that has the copper rolled up, an orange-colored dissolution begins to form, while nothing happens in the vicinity of the nail with the zinc.
What we are observing is the formation of ferric oxide on the surface of the nail with the coiled copper. In the following images we can compare how the solution with the ferric oxide is colored as the time of the experiment passes.
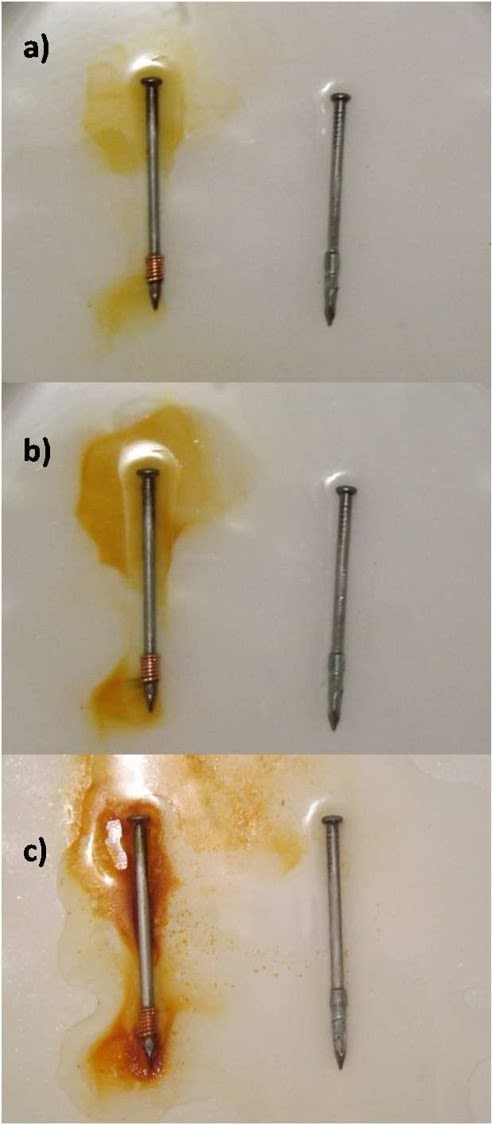
Progress of oxidation in the experiment: a) 4 hours, b) 12 hours and c) 24 hours. Source: @emiliomoron.
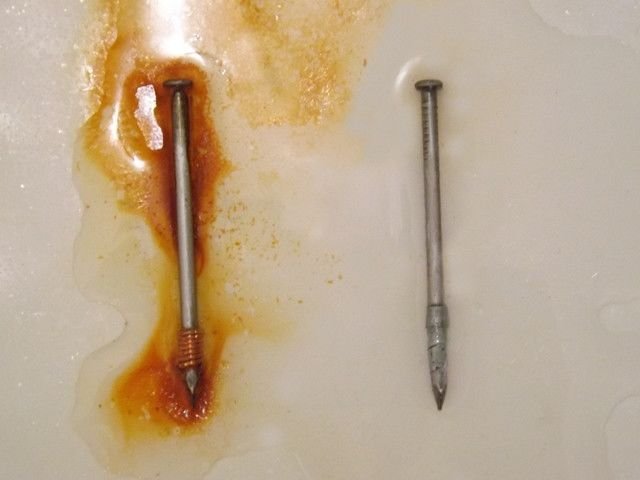
As we can see the orange dissolution only becomes greater in the vicinity of the nail with the copper, however we do not notice this effect around the nail with the zinc. In this case we are only witnessing the oxidation of the nail with the copper, so the zinc offers protection to the other nail.
On an iron element, such as a nail, anodic and cathodic zones occur, in the anodic zones iron oxidation occurs and in the cathodic zones oxygen reduction, the overall reaction is described as follows:
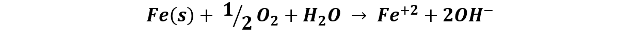
The iron ions then react with the dissolved oxygen in the water to produce ferric oxide, Fe2O3, which is responsible for coloring the solution and the surface orange-red.
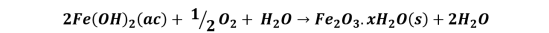
These reactions also take place on the nail with the copper wire, only that, the cathodic zone is located preferably on the copper element, reason why the reduction of the oxygen will take place in this place; whereas the anodic zones are located throughout the nail, producing the oxidation of the iron. If we look at the table of reduction potentials, copper is a less active metal than iron (more noble) so the iron has more facility for corrosion than this, so basically the copper element resists corrosion and rather causes it in the nail.
On the other side, in the nail that has the piece of zinc bonded, there are no signs of corrosion, in contrast to what happened with the other nail, zinc is the element that suffers corrosion because it is a less noble metal than iron, due to its reduction potential is lower, so in this case, it is on the zinc where the anodic areas are located, and it is the one that suffers oxidation, while the nail acts as a cathode, inducing the reduction of oxygen and thus saving itself from corrosion.
The oxidation of the zinc that takes place on the anode is described according to the reaction:
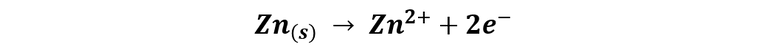
In the following image we can compare the aspect of both nails after removing from the solution and drying:

Comparison of the nails of the experiment: with zinc (right) and with copper (left). Source: @emiliomoron.
In summary, the sacrificial anode protects a metal by oxidizing and causing the protected metal to shrink, preventing it from rusting. Just as we have seen the protection of the pin thanks to the zinc is produced because the pin becomes the cathode of the electrolytic cell formed by both metals and the electrolytic solution, therefore it is known as cathodic protection.
With this simple experiment we can also demonstrate that not just any combination of metals will work for the purpose of protecting the iron, because, as we have seen with copper bonded to the nail, the effect is counterproductive, rather we induce the corrosion of the iron.
Well friends, until here the present post, I hope you liked the experiment and the information is useful, thank you very much for reading. See you next time!
Reference
- Chang, R. (2002). Chemistry. 7ma, McGraw-Hill.
- Wikipedia.com. Cathodic protection
Educative. Do keep up the good job.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Thanks for using the STEMsocial app and including @stemsocial as a beneficiary, which give you stronger support.