Turmeric extract for more efficient and environmentally friendly ethanol fuel cells
The global energy market demands reliable energy sources with low environmental impact, which has led to focus more interest in fuel cells to supply energy in stationary and mobile applications, however, the safety issues associated with the use of hydrogen fuel cells, due to the high flammability of this gas, in addition to the fact that the hydrogen used is derived from fossil sources have limited the implementation of this technology; that is why many researchers have put the interest in ethanol, as a substitute in fuel cells, but this requires the use of more efficient catalysts.
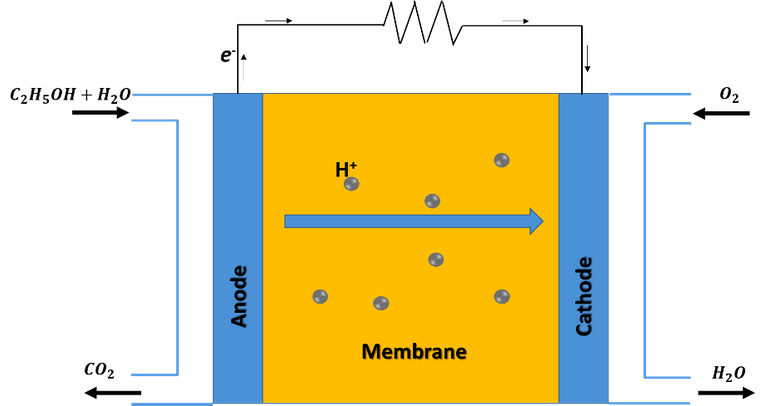
Schematic diagram of an ethanol fuel cell operation. Source: image created in powerpoint.
Ethanol fuel cells are considered a sub-category of the so-called proton exchange membrane fuel cells, in which the chemical energy of ethanol is converted into electrical energy through the oxidation reaction of ethanol on the surface of a catalyst. The catalyst acts as an anode and hydrogen is extracted on it, which crosses a solid polymer membrane that acts as an electrolyte, in the process electrons are released and travel from the anode to the cathode, generating electric current; then, the protons that cross the electrolyte meet at the cathode with the oxidant, generating water and carbon dioxide as by-products of the process.
Reaction at the anode:
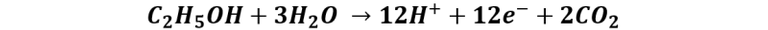
Reaction at the cathode:
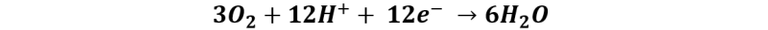
Thanks to this technology it is not necessary to store hydrogen to produce energy, but the reaction is very slow without the appropriate catalyst, so the challenge is to find a more active and efficient anode catalyst for ethanol oxidation.
Curcumin for super-efficient ethanol oxidation
Now, curcumin is one of the substances present in turmeric, a natural colorant and spice widely used in cooking, being specifically a diarylheptanoid, a naturally occurring phenol. And this cooking spice can perhaps provide us with safer and more efficient fuel cells.
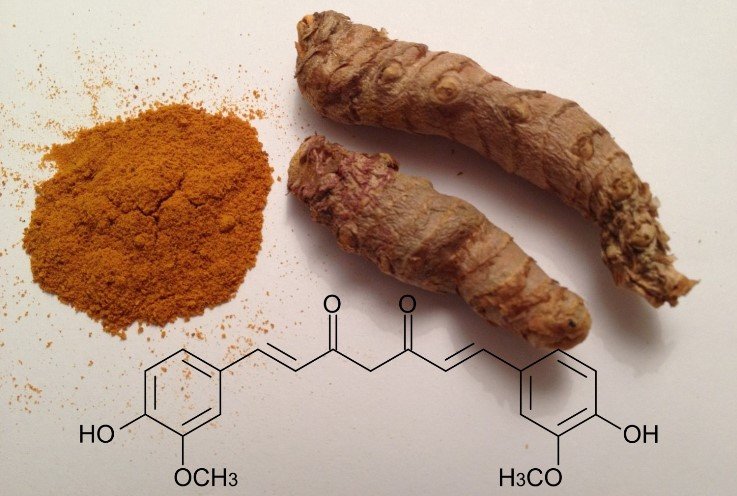
Chemical structure of cucumin. Source: original edited image from pxhere.com.
A group of researchers from the Clemson Nanomaterials Institute (CNI) and collaborators from the Sri Sathya Sai Institute of Higher Learning (SSSIHL) in India discovered that by combining this substance with gold nanoparticles they had an electrode that is 100 times more efficient at converting ethanol into electricity.
Conventional fuel cells use platinum as a catalyst at the anode of the cell, but platinum is poisoned by the reaction products and also raises the cost of the fuel cell, so the researchers focused on optimizing the anode of the cell, since that is where the ethanol oxidation takes place.
And to optimize the anode, the researchers used gold nanoparticles, as they have ideal properties for catalytic reactions in many applications. But this requires the synthesis of highly stable and ultra-small nanoparticles, and these researchers, instead of using metal-organic frameworks, polymers or other complex material to deposit the nanoparticles, used curcumin for its particular structure.
The researchers presented in their work a galvanostatic synthesis route to create gold-curcumin nanocomposites, which basically consist of gold nanoparticles smaller than 2 nm wrapped by a curcumin porous network (AU-CM). In the research they found that for the deposition of the nanoparticles on the surface of a vitro carbon electrode they used an electric current 100 times lower than with other catalysts, and that without the curcumin coating, the gold nanoparticles agglomerate, reducing the surface available for oxidation reaction, obtaining a very poor performance. The results of this work were presented in April in the journal Nano Energy.
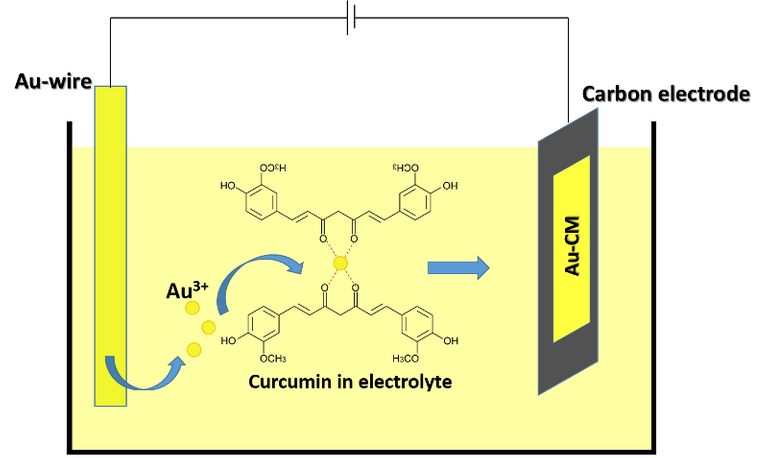
Schematic of electrodeposition of gold-curcumin (Au-CM) nanocomposites on a glassy carbon surface as electrode. Source: image created in powerpoint.
Among the results obtained, it also stands out that the nanocomposite obtained showed excellent stability, easy electron transfer capacity, and high catalytic activity for the electrooxidation of ethanol and methanol in alkaline media, characteristics that were compared with the best gold-polymer catalysts.
Although the research is still at an early stage and more tests need to be performed to scale up the work to industrial scale and build fuel cells for real applications, curcumin showed that it can stabilize and create a porous medium around the gold nanoparticles leading to excellent performance in alcohol oxidation, which could bring hydrogen fuel cells closer to replacing hydrogen, and produce more efficient fuel cells for vehicles, buildings, electronic devices and power systems, without the dangers of storing hydrogen and using ethanol, a fuel that can be obtained from biomass fermentation, making the process more environmentally friendly.
Thanks for coming by to read friends, I hope you liked the information. See you next time.
References
Sai Prasad Nayak et al(2022). Green synthesis of a novel porous gold-curcumin nanocomposite for super-efficient alcohol oxidation; Nano Energy; Volume 94.
Wikipedia.org. Fuel cell
Wikipedia.org. Curcumin.
Best of all, companies that want to make fuel cells that look like they contain turmeric can use yellow dye number 2.
Hi @yintercept, I think the yellow dye number 2 is a synthetic dye, I would not know if it can also be used as in the case study.
My post was a really bad joke. It is common for manufacturers to replace turmeric with yellow dye number 2.
It is a good example of how they take something healthy out of food and then replace it with something that is potentially carcinogenic to increase profits.
For example, most pickles in the United States use yellow dye number 2. The dye make it look like they added turmeric.
If there are batteries that use turmeric; I suspect that we will see the same thing. There will probably be companies that remove the turmeric and replace it with the dye to make it looks like it works.
OKOk... I understand, certainly there are those who then want to take advantage and replace the components with something cheaper, but here it would not work for them because of the difference between the molecules hehe.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thanks for the support my friends!