The esters and their smells
As you know, there are synthetic substances that are added to food to increase its smell and taste. These are what we know as artificial essences. Well, within this type of substances there is a chemical species that stands out for the variety of pleasant odors that they present, so they are widely used as flavors, I speak of the esters. Many of the artificial essences that we can find on the market contain molecules of this type of chemical compound, which in general are famous for giving off a pleasant fruity smell, but what is most surprising is how you can prepare different essences by simply changing the number of carbon and hydrogen atoms in their molecular structure.
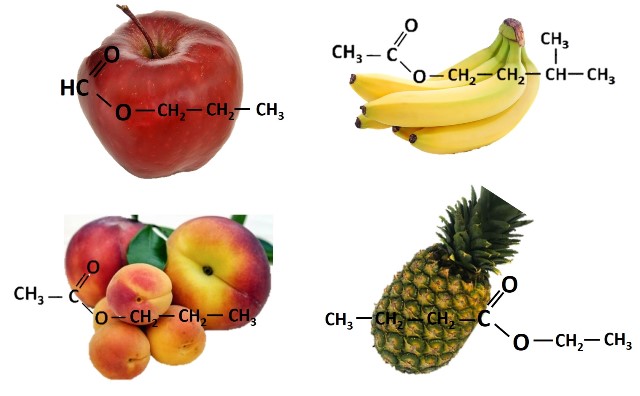
Many esters have a fruity smell. Source: @emiliomoron, design elaborated with images of public domain.
Let's define what an ester is
The term ester refers to an organic compound derived from carboxylic acid in which the hydrogen atom of the hydroxyl group has been replaced by an alkyl group. The general structure is the product of the union of the function of carboxylic acid (the R group) and the carbon chain that forms the alcohol (the R' group)[1]. The general formula of an ester is expressed as follows:
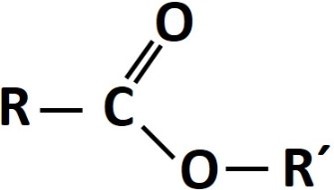
In the structure shown, the R group can be a hydrogen atom or a carbon chain, while the R' group can only be a carbon chain, since a hydrogen atom would make the structure correspond to that of carboxylic acid.
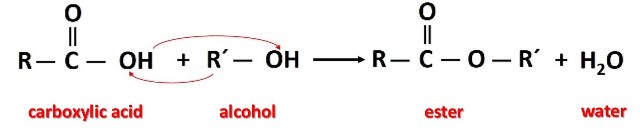
Esters can be obtained by the reaction between alcohols or phenols and carboxylic acids in condensation reactions. The general reaction is shown in the following image.
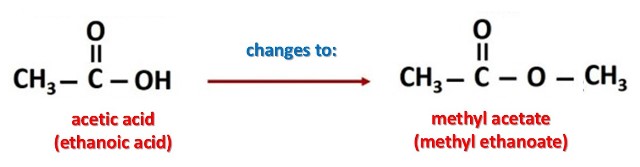
Naming the esters is very easy, they are named after the carboxylic acid from which they come; the names of carboxylic esters include prefixes that denote the lengths of the carbon chains in the molecules and are derived following rules of nomenclature similar to those of inorganic acids and salts, for example:
Its aromas
The lower members of the esters of carboxylic acids are colorless, fruit-scented liquids, while the upper members are odorless.
With reference to the first ones, different combinations of alcohols and carboxylic acids give rise to different esters, and each ester has a unique aroma. And it is very interesting how with only small differences in the number of carbon atoms in the acid function of the ester or in the group provided by the alcohol, such significant differences in the smell of the molecule can be produced.
In the following image we can observe how with the simple substitution of the R' group or the quantity of carbon atoms in the acid group the aroma of the resulting compound changes.
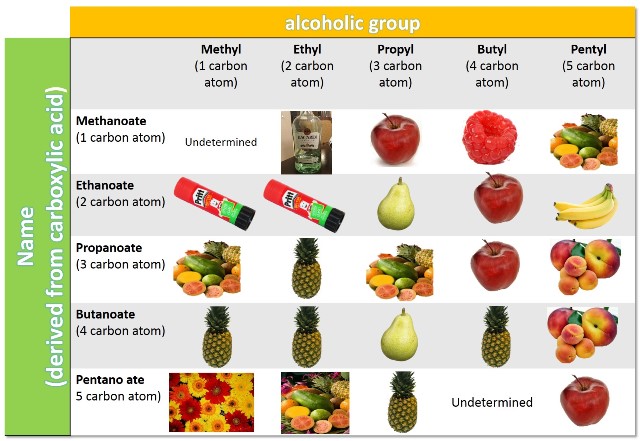
Source: @emiliomoron, composition with images of public domain
All of these esters are edible in small quantities and are naturally found in all fruits, vegetables, herbs and spices. As well as in most natural fats and oils, which are made up of the fatty acid esters of glycerol.
Characteristics and properties
Esters are polar molecules, which is due to the presence of the C=O group, which gives the polarity to all compounds derived from carboxylic acids[2]. However, their boiling points are lower than those of carboxylic acids and alcohols of similar molecular weight, essentially because there is no intermolecular interaction through hydrogen bridges between the molecules of the esters. For example, let's compare the boiling point (B.P.) of ethyl acetate with that of two compounds with the same molecular mass (M.M.).
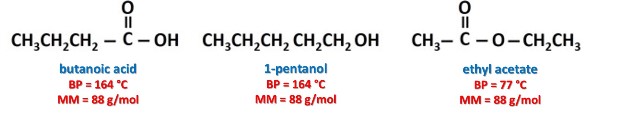
But, esters can form bonds by means of hydrogen bridges through their oxygen atoms with hydrogen atoms of water molecules, which makes esters slightly soluble in water. However, since esters do not have a hydrogen atom that forms a hydrogen bond with an oxygen atom of the water, they are less soluble than carboxylic acids.
These characteristics make the esters volatile, even more volatile than the components from which they are derived. In plants it is a determining factor in being able to spread their aroma and thus attract a predator or some pollinating insect, and in industry it is especially desirable in the manufacture of perfumes and flavors, although in the food industry this volatility is a problem.
Another interesting characteristic is that they are easy to synthesize through an esterification reaction, but it is interesting because the aroma of the ester obtained is sweet and fruity, unlike the carboxylic acid from which they are derived, which can be quite unpleasant in some cases. Let us see two examples of this interesting characteristic:
1- Let's note the differences that occur when we combine the same carboxylic acid with five different alcohols. Let's take the acetic acid and keep the acid part constant in the molecule.
Table n°1. Esters derived from acetic acid with various alcohols
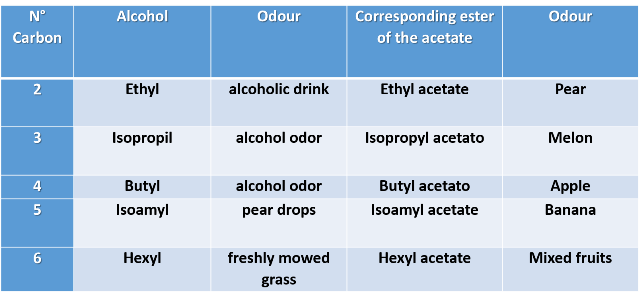
2- Let's do the opposite, keep the alcohol part constant and combine with 5 different carboxylic acids. Let's use the ethyl alcohol as an example.
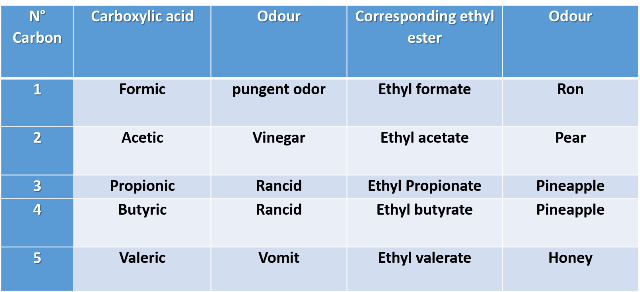
In this last example it is clearer that carboxylic acids have an unpleasant odor, while the odor of the esters that are derived from them is quite the opposite.
So why do esters smell so good?
There could really be a great number of reasons or only one, but it is still not entirely clear; it seems to be due to a genetic advantage produced in plants so that certain species eat their fruit; and thus spread the seeds thus allowing new plants to grow and colonize other places. Or it could be the other way around, that animal species have evolved to recognize that certain sweet smells and tastes mean that there are good foods, so we associate the smells of these molecules as pleasant.

Animal species evolved to recognize certain odors. Source: pxfuel.com, CC0
And how have esters helped the processed food industry?
Our society has a great demand for food, so it is necessary to resort to processed foods to satisfy that demand. Even so, when we buy a canned product we expect this food to have the appearance of a fresh food; but as we mentioned, due to its low boiling point, the esters that naturally give food its aroma are usually lost during processing when they are heated, and we know that smell is fundamental to perceive a pleasant flavor, so, in order to make processed food more attractive, the same esters that are lost during processing must be added later.
Although in some cases the evaporated esters are recovered and added again, in other products this is not possible; therefore they are added in a synthetic way. And whether they are natural or synthetic, the ester molecules are identical, so unless it is labeled on the can, we will never know that the odor was added.
![20201019_091935[1].jpg](https://images.hive.blog/DQmdeb9fLxMM4iBDVC7R1oMb5aYi1RCwUbx8mCFWhTpUbEH/20201019_091935[1].jpg)
In the label of the products we will be able to read if it has added aromas. Source: @emiliomoron.
Well my friends, I hope you liked the post, and now look at the label to see if the product you bought has as natural a smell as the packaging says it has. See you next time!
References
1.Chemlibretexs.com. Carboxylic Acids and Esters.
2.Morrison, R., Boyd, R. (1996). Organic Chemistry. Fifth edition, Addison-Wesley Iberoamericana.
Source of the images:
White rum, CC BY-SA 2.0; apple, CC0; mixed fruits, CC0; pineapple, CC0; banana, pixabay licence; blackberries, strawberries, cherries, pixabay licence; flowers, CC BY-SA 2.0; pear, pixabay licece; glue, CC0.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Your post has been voted as a part of Encouragement program. Keep up the good work!
Dear reader, follow and support this author, Install Android: https://android.ecency.com, iOS: https://ios.ecency.com mobile app or desktop app for Windows, Mac, Linux: https://desktop.ecency.com
Learn more: https://ecency.com
Join our discord: https://discord.me/ecency
Excellent delivery, very complete the information that you share with us about esters, friend @emiliomoron it is inferred that the waxes that agro-industrial companies use to give showiness to the fruits are precisely based on esters, in the case of the apple it has been inferred that this small film that gives it that brilliant shade, is carcinogenic.