Protection of steel by galvanizing
We all agree that steel is one of the materials with more applications in our daily life, it would be very difficult to conceive many articles and structures without this versatile material; from buildings and bridges to household items, and we also know that it is a material with a very big tendency to rust, creating that unattractive oxide layer that with time deteriorates the material. That is why we must resort to different ways of protecting it to extend the life of the elements and structures made of steel, these ways range from adding layers of paint or coating it with other metals, and this last one is what I want to talk about in this post, how the galvanic coating protects the steel from corrosion.

Galvanized steel is present in many structures. Image credit: pixabay.com.
We can consider galvanizing as the process of coating steel with several layers of zinc, which gives it not only a better finish, but also a protective layer against oxidation.
Let's remember why iron oxidizes
We know that any element of iron (including steel) when exposed to the environment oxidizes and deteriorates; this is what we know as corrosion, which is the reaction suffered by metals in contact with the air and moisture present in the environment.
Iron, and other metals, usually corrode through an electrochemical process involving the flow of electrons. Therefore, four components are necessary for the process to take place:
- Anode (negative electrode)
- Cathode (positive electrode)
- Metal conductor
- Electrolyte (which can be water or a solution)
On the surface of the steel, there are small irregularities or discontinuities in the composition in which small differences in electrical potential are established, resulting in the formation of areas that function as small electrolytic cells composed of anodes and cathodes, the following image shows a representation of one of these cells.
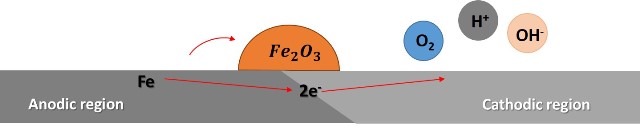
Representation of iron oxidation. Source: @emiliomoron.
Then, the surface of the steel works as an anode (where oxidation occurs) and the metallic iron loses two electrons and changes its oxidation state to Fe(II), according to the following reaction:
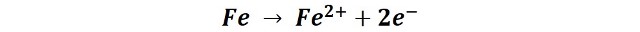
And the region adjacent to the steel works as a cathode, and in this region the electrons of the iron are transferred to the oxygen:
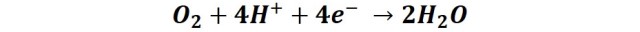
As a result of the difference in potential established in the small cell, electrons flow from the anode to the cathode, and the iron atoms in the anode region are converted to cations (or positively charged ions); these cations attract and react to form the oxide, the overall reaction can be described as follows:
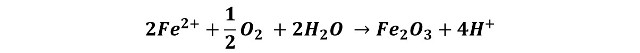
And as the anodic regions corrode the material, the oxide layer breaks off and the areas that are not corroded will now be attacked, and the process will continue until all the steel is consumed.
That is why we need to protect all the elements made of iron from the elements of the environment, and galvanizing offers two forms of protection:
Ways in which galvanizing protects the steel
Barrier protection
As we can see, the oxidation of the steel begins on the surface of the element, so one way to prevent this from happening is to apply a barrier that covers the metal piece. In this case is when we apply a coat of paint that provides a waterproof protection on the item. However, there are elements where applying a coat of paint is not possible, such as screws, or when we want to give a better finish to the steel.
Then, it is when we recur to galvanizing; this is an electrolytic process that consists of coating one metal with another, the function of this layer of metal being to protect the surface of the one on which it is deposited. The most common galvanizing process consists of depositing a layer of zinc on iron, and since zinc is more oxidizable than iron, it provides a protective layer by developing a stable patina on the surface of the material. This patina is composed of oxides, hydroxides and some zinc salts, depending on the medium where it develops. And once this patina is stabilized, it provides protection to the galvanized coating as well, thus considerably reducing the corrosion process.
The zinc patina begins to develop when the coating is exposed to the environment, forming a layer of zinc oxide on the surface of the material.
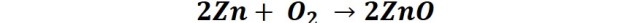
Later, this oxide layer reacts with water from air humidity or rain giving rise to zinc hydroxide, which in turn reacts with the carbon dioxide contained in the air, thus forming an insoluble and strongly adhering film.
Representation of the formation of the protective patina on the steel Source: @emiliomoron.
The advantage of this protective coating over the paint layer that we usually add to protect metals, is that conventional paint is degraded by the action of solar radiation and is released by impact or abrasion, leaving the base material unprotected, which can advance corrosion even by a small unprotected area, which even allows corrosion to advance under the paint layer without being detected. On the other hand, galvanizing does not deteriorate under the action of the sun, and if the protective patina is detached by mechanical action it is restored again as long as there is zinc on the surface.
Protection against galvanic corrosion
Galvanic corrosion is an electrochemical phenomenon that occurs when two different metals in direct contact are immersed in an electrolytic medium or wet environment; if these metals have different electrode potentials, the less noble metal tends to corrode faster protecting the other metal. It is something very common that we see when common screws are joined to stainless steel sheets, we observe an accelerated corrosion of the screws. In this case, it is said that the less noble metal (the one that oxidizes faster) is used as a sacrificial anode, and in many structures this property is taken advantage of by installing a metal that suffers the attack of corrosion instead of the metal that we are interested in protecting.
One advantage of galvanizing is that it also functions as a sacrificial protection system. When the zinc coating is cracked or broken by a cut or damage to the surface, the small areas of steel that are exposed are protected from corrosion by the surrounding coating, no touch-up or repair is necessary.
In the presence of an electrolyte (such as a wet environment or seawater), the zinc coating and the one-piece galvanized steel develop electrical potential differences, thus forming an electrolytic cell; and because zinc is more electrochemically active, it becomes the anode of the cell, rather than small anodic and cathodic areas forming on the surface of the steel.
It corrodes preferentially to the base steel, preventing corrosion of the small areas that may be exposed; and this sacrificial protection will continue as long as some of the surrounding coating remains.
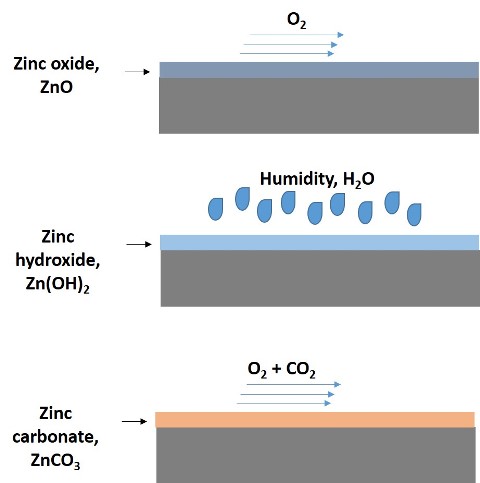
Galvanic series of some metals in sea water as electrolyte. Source: series adapted from wikipedia.com.
According to the galvanic series shown in the previous image, the higher up the series, the nobler the metal is and the more it is able to corrode those below it in the series. Thus, since zinc is lower in the series than steel, this is who suffers the attack of corrosion.
Mechanism of cathodic protection
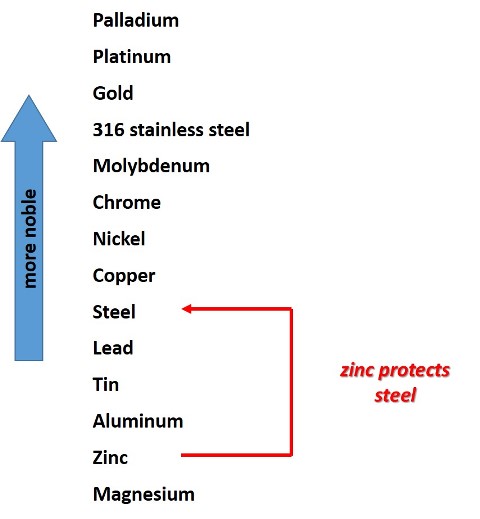
When zinc and steel are in contact in an electrolytic medium, a potential difference is established between the two metals and a galvanic cell is formed. And since zinc is more active (as shown in the galvanic series) it assumes the role of the anode in this cell.
Forming an electrolytic cell in the galvanized steel immersed in an electrolyte. Source: @emiliomoron.
Then, as a product of this potential difference between the two metals, electrons flow from the zinc (which acts as the anode of the cell) to the steel (cathode), and the zinc atoms are oxidized to Zn(II).
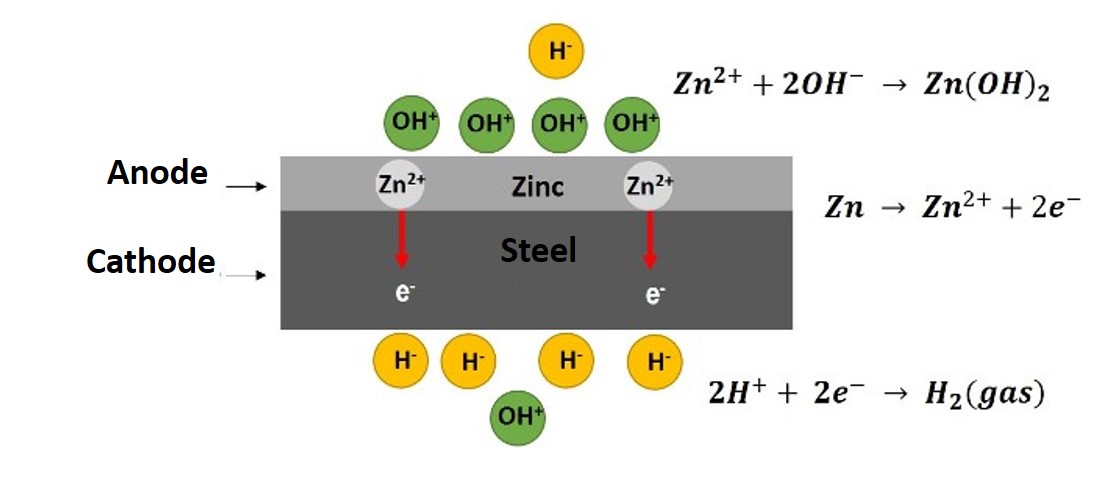
Diagram of the mechanism of cathodic protection Source: @emiliomoron.
These zinc cations on the surface attract and react with the hydroxyl ions present in the electrolyte, forming zinc hydroxide as we have already mentioned, slowly consuming the zinc but at the same time providing the protective layer.
On the surface of the steel (cathode) the electrons attract and react with the hydroxyl ions of the electrolyte, releasing hydrogen gas; so there is no reaction between the steel and the electrolyte, that is, the cathode is protected by the reaction at the anode, which is known as cathodic protection.
When a discontinuity in the zinc coating occurs as a result of a scratch or blow, and exposes the base steel, the cathodic protection that the zinc provides to the steel comes into action and ensures that the exposed steel does not rust.
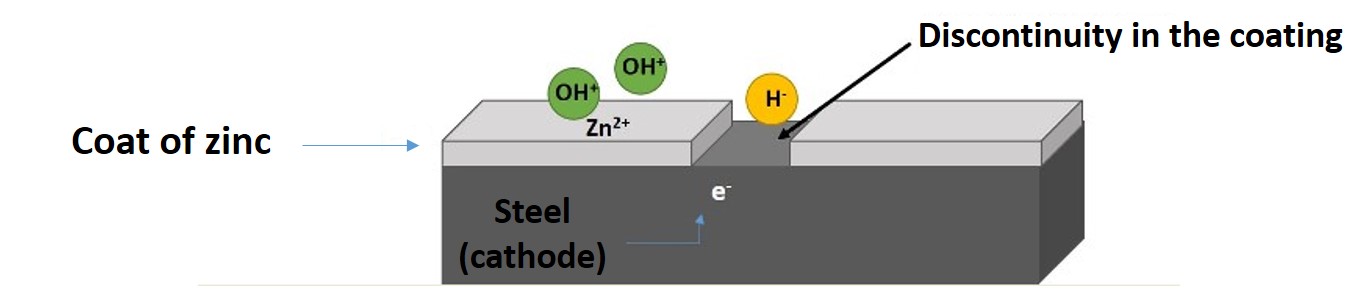
The negative side of galvanizing is that the protective patina provided by zinc is susceptible to dissolution when the medium is sufficiently wet and acidic, so care must be taken when choosing its application, since as a sacrificial protection the zinc will be consumed and the base material will be reached by corrosion.
As you can see, metal coatings help us to provide steel with much-needed corrosion protection; and in this, zinc coating as sacrificial protection offers an unmatched ability to provide both barrier protection and cathodic protection in exposed areas, resulting in an excellent combination between the strength of the steel and the long life provided by the zinc coating.
Well friends, that's all for now, I hope you liked the information and it is useful, thanks for reading, see you next time!
References
Wikipedia.com. Corrosión
Wikipedia.com. Serie galvánica.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Thanks my friends!
Your post has been voted as a part of Encouragement program. Keep up the good work!
Try https://ecency.com and Earn Points in every action (being online, posting, commenting, reblog, vote and more).
Boost your earnings, double reward, double fun! 😉
Support Ecency, in our mission:
Ecency: https://ecency.com/proposals/141
Hivesigner: Vote for Proposal