New biofuel made from bacteria could power rockets
As we are well aware we must strive to decarbonize our energy system to slow the accelerating pace of climate change, and while it is true that great strides have been made in the electrification of light vehicles, the decarbonization of the power grid and the use of biofuels to reduce our dependence on fossil fuels, much remains to be done to electrify or find a substitute for fuels for heavier applications such as land and sea freight, transatlantic aviation and rocketry.

sectors such as aerospace are still dependent on fossil fuels. Source: pxhere.com.
Since long-haul heavy-lift transportation and aerospace rely on high energy density fuels derived from petroleum, such as Jet-A for aviation or fuels like RP1 or HTPB used in rocketry, it is difficult to find a biofuel to meet their high weight/power requirements. These fuels are rich in cyclic and branched alkanes or synthetic polymers, and these have been difficult to replace from these applications as they offer high energy density, the problem is that they release a large amount of COx, NOx and SOx gases when burned.
But a group of biofuels researchers led by Lawrence Berkeley National Laboratory was inspired by the biology of a rare antifungal molecule made by a bacterium to develop a biofuel based on naturally occurring cyclopropaned molecules with an energy density comparable to the rocket fuels currently used by NASA.
Cyclic alkanes have special characteristics that are conferred by ring tension, especially when the carbon-carbon bonds have angles that deviate from 109.5°, the normal angle for bonds between atoms with hybrid sp3 orbitals. And the deformation of these bonds causes high heat of combustion, a property that has been exploited to produce fuels with high energy density.
For example, in cyclopropane (CP), the simplest of the cycloalkanes, which consists of a ring formed by the bonding of three carbon atoms, bond angles of 60° are formed between the carbon atoms in this molecule, a very small angle compared to the normal angle of 109.5°. And it is precisely this bond angle that gives cyclopropane the highest stress and heat of combustion among the cycloalkanes.
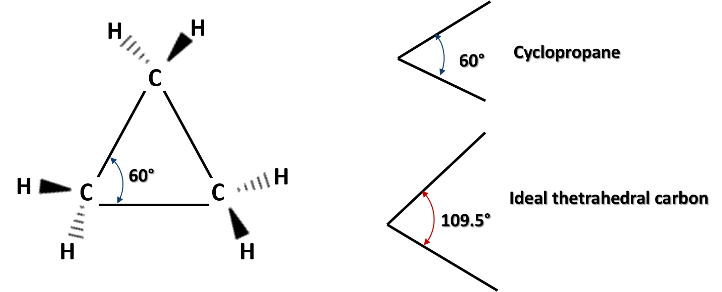
Structure of cyclopropane ring. Source: image elaborated in powerpoint.
And this high heat of combustion of cyclopropanes was used to make Syntin fuel, a synthetic fuel developed during the Soviet era to power the upper stages of Soyuz and Proton rockets.
But this group of researchers managed to produce a biofuel based on polycyclopropane fatty acid methyl esters in bacteria, called POP-FAME in the research, and this biofuel can reach an energy density of more than 50 MJ/L, a value higher than that found in the most commonly used jet and rocket fuels today.
To produce the biofuel, the researchers focused on exploring organic compounds with rings formed by three carbon atoms produced by microorganisms, finding that to date only two natural molecules have been found that include multiple cyclopropane rings, the antifungal FR-900848 also known as jawsamycin and the cholesterol transfer protein inhibitor U-106305, both molecules are produced by the Streptomyces battery, and fortunately, the biosynthetic pathway of jawsamycin has now been elucidated due to interest in its antifungal properties.
The natural antifungal FR-900848 is named jawsamycin because of the characteristics of this molecule; it contains five cyclopropane rings that give it the appearance of a jaw full of teeth. And the authors of the research studied the genes of the Streptomyces bacteria that encode the enzymes responsible for building the teeth of the molecule, and found a combination of enzymes that allowed them to manufacture the molecule with the characteristic jagged rings but skipping the other parts of the chemical structure of the molecule.
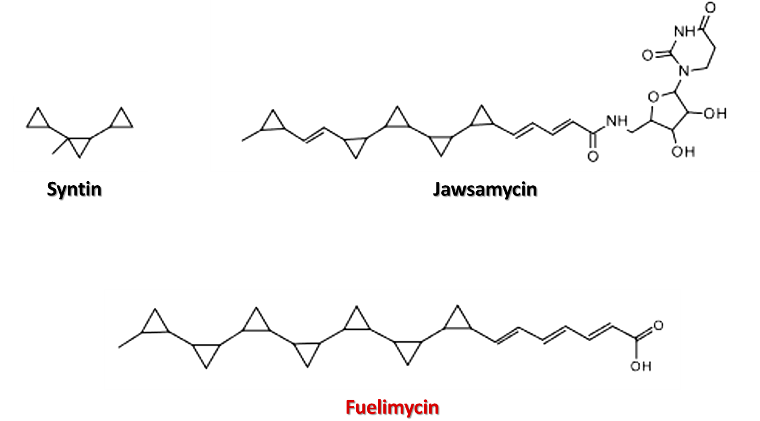
Comparison between the structures of cyclopropanate molecules. Source: image elaborated in powerpoint.
In this way, they were able to produce molecules containing up to seven cyclopropane rings linked to a backbone of carbons that they called fuelimycins, and according to their calculations, the energy density of fuelimycins is comparable to that of RJ-5, a petroleum-derived rocket fuel.
Unfortunately, working with bacteria is not easy, and they do not always cooperate to work in the laboratory. As far as productivity is concerned, there is still much work to be done, but the research was able to demonstrate that the molecules functionalized with cyclopropanes by biosynthetic means were excellent fuels and that it is possible to produce biofuels with high energy density.
Well friends, I hope you liked the information and let's hope that soon fossil fuels can be replaced in all their applications. See you next time!
References
Wikipedia.org. Ciclopropano
Wikipedia.org. Cicloalcano
Pablo Cruz-Morales et al. (2022). Biosynthesis of polycyclopropanated high energy biofuels. Joule 6, 1–16
Interesting i didnt know about this fuel! Thanks
!1UP
Click this banner to join "The Cartel" discord server to know more.
Hello friend, certainly interesting.
You have received a 1UP from @gwajnberg!
@stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
Thanks!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thanks for the support friends!
When it comes to decarbonization, I find it very hard to see batteries solving the problem. While it could work for cars, working for rockets and planes that uses Jet A-1, JP-5 and other Aero turbine kero, will be very difficult due to the mass of batteries needed to give such power, but I have always seen fossil fuel has a hopeful light, and while microorganisms can be very difficult to work on for fuel, knowing that difficult to work on for fuel, seeing researches like this, gives me hope.
That's right my friend, fossil fuels are difficult to replace for power hungry applications, hopefully this type of research will pave the way for more environmentally friendly substitutes.
This is an amazing news. Thanks for sharing it with us.
I am nevertheless afraid that we are still far from any practical applications (for the reasons you mentioned: we need to massively produce such a fuel so that it could be used on a daily basis).