Copper patina, an attractive corrosion phenomenon
You may have noticed that the monuments made of copper or bronze that rest in squares and public places develop a curious green coating, this effect can also be seen in the old buildings of many cities. Well, it may not seem very curious but this effect is produced by the natural corrosion of copper.

Green patina on some monuments. Source: trumpet player and Minneapolis City Hall, public domain images.
What is interesting is that a phenomenon as undesirable as the corrosion of metals can produce such a decorative patina, which is even highly desirable. This bluish-green patina on the surfaces of copper or its alloys is a layer of copper salts produced by the corrosion process experienced by this metal due to the action of chemical agents present in the atmosphere, since, like other metals, copper oxidizes due to the natural tendency it has to return to its mineral state; that is, in the form of rust. So, when exposed to the environment they slowly combine with some elements found in the air to return to their natural state. The particularity of copper, is that this patina formed by the process of corrosion, then protects it from the same, preventing the metal from deteriorating over time, thanks to this are so durable our heritage and at the same time look elegant beauty, because this patina is changing color over time and is unique to each place due to its different climatic conditions, so the beauty of copper and its alloys is unique among decorative metals.
In this way, although copper does not maintain its metallic shine, it is a metal that is adorned with a beautiful patina that can even tell its story. Knowing how this protective layer is formed will give you another vision of the changes that occur throughout the history of our monuments or some family memory. That's why in this post I want to comment on how the natural corrosion process of copper gives us this colorful patina.
How is this green patina formed on copper?
The formation of the copper patina is very similar to the formation of iron oxide, both occur as a result of the interaction of the metal's atoms with the oxygen in the air in the presence of water.
Copper reacts with air to form copper dioxide (reaction 1), which then reacts with more oxygen to form copper oxide (reaction 2).
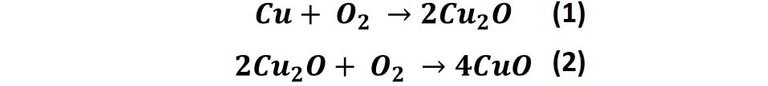
This copper oxide formed in reaction 2 is responsible for the later formation of the patina colors. Since there is more than oxygen in the air we breathe, copper's reactions are not limited to these two. For example, when copper oxide reacts with carbon dioxide in the air, copper carbonates are produced in a pair of reactions that generate the color of the patina on the metal [1], that is:
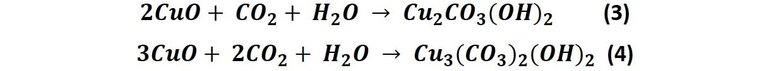
In reaction 3 a compound called malachite is produced (Cu2CO3(OH)2), specifically it is the the copper(II) carbonate hydroxide, formerly widely used as a dye [2]; this compound that varies from dark green to blue, in reaction 4 a compound called azurite is produced (Cu3(CO3)2(OH)2) this is a compound that varies from blue to purple tones. Although both compounds are basic copper carbonates, the difference in colors is due to the different oxidation states of the metal in both chemical formulas [3].
Another reaction that can influence the color of the patina is that which occurs when the copper oxide reacts with the sulfur oxide found in the air as a result of the combustion of fossil fuels. This produces the following reaction:
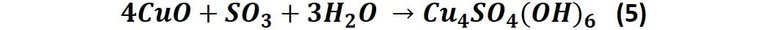
The compound Cu4SO4(OH)6 is known as Brochantite, and is emerald green in color.
That is why the patina produced on the sculptures exposed to the environment can have a color that varies between green and blue tones. And that is why I was telling you that the patterns of the patina depend on the history that each copper object has had. Thus, the products of the above reactions have their own color hue, and the combination of them describes a unique feature of the patina. For example, the darker greens indicate an abundance of malachite and broprantite, the product of an environment with high concentrations of CO2 and SO3.
Then, as we see, the patina is produced on the copper as a result of chemical reactions, the more compounds interact with the metal the more products are formed, there are several factors that influence the speed of patina formation and the color it adopts, but generally, typical of the type of alloy and the environment, but generally it is produced as we have established.
However, when different compounds are mixed on the surface of the copper object, a pleasant shine of colors is produced, sometimes several between turquoise and emerald, which make this patina an attractive decorative effect very sought after.
How to create your own patina
As I was saying, in jewelry and ornaments, the blue and emerald tones of this patina is very sought after as a decorative effect in copper pieces. That is why we can try to reproduce this patina that develops naturally, but in practice it needs to be developed quickly. So let's see how we can do it.
Procedure and results
Experiment n° 1. Immersion method
This experiment is very simple, we will only need a coin covered with copper, white vinegar and a little salt. In a container we will add a little vinegar with a pinch of dissolved salt, and we will leave the coin soaking for a few hours.
Soon we will begin to observe that the coin is getting darker, losing its metallic shine, and that greenish areas also begin to appear. In the following images I show you the advance of the reaction on the soaked coin.

Image of how the patina is produced on the coin immersed in the solution. Source: @emiliomoron.
As we can see, the coin is changing to a dark tone, a sign that it is oxidizing, and then there are greenish stains that spread over the entire surface of the coin. What we are seeing is the formation of a blue or green patina known as verdigris[4], which, unlike the patinas described above which are derived from copper carbonates, is produced mainly by the appearance of copper acetate (II).
Then, this patina that we are generating in a fast and artificial way, if you want to say, is a consequence of the action that acetic acid (vinegar) has on copper, specifically on the copper oxide that is produced in reaction 2, the process can be described by the following reaction:
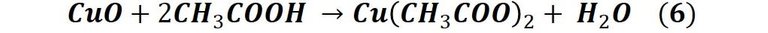
In the following image we can see how the coin looks once it is removed from the solution and left to dry in the sun.

Appearance of the patina on the coin after removing it from the solution and allowing it to dry. Source: @emiliomoron.
As we can see, once it has been allowed to dry, the greenish color of the patina is much better appreciated, giving us that aged look on the coin.
Experiment n° 2. Electrolytic patina
Artificial patina on copper objects can also be achieved by an electrolytic process using direct current. An electrolytic bath is used for this purpose, with the object to be treated acting as the cathode of the cell. A simple assembly is described in the following figure.

Electrolytic patina. Source: @emiliomoron.
The electrolytic solution to be used should contain copper ions together with ions that facilitate the transport of the electric current. In the experiment, the combination of two salts, copper (II) sulfate pentahydrate and potassium dichromate, was used. The electrolytic bath was operated at room temperature with a voltage of 9 V, giving a green patina, as shown in the following image.
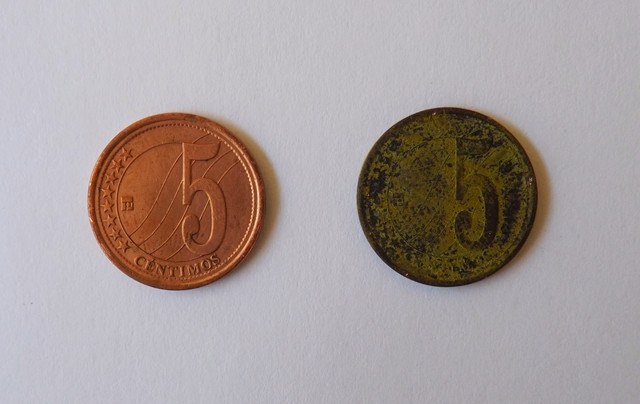
Copper coin, before and after the electrolytic bath. Source: @emiliomoron.
Conclusion and contribution
Well friends, as we have been able to understand, the formation of the copper patina on the pieces of copper is due to a chemical process, which corresponds to a set of reactions that take place between the copper oxide that occurs naturally on the metal by its contact with oxygen in the air, and the subsequent reaction of this with some compounds present in the environment, so their colors and patterns depend much on the quality of air present, so there are several factors that depend on the speed with which it forms and the color it adopts.
The shapes described here to recreate the marshmallow patina serve as a guide and can be used to decorate ornamental copper pieces, reproducing that aged look in a short time. I believe that with some practice and patience, truly artistic patterns could be achieved, controlling the thickness and pattern of the patina with the way the solution is applied, but your humble servant limited himself to exploring the chemistry of the process.
These patinas give us a really amazing aspect in the copper objects that we use as decoration or in our monuments, maybe more than once we have appreciated that aged aspect of our sculptures thinking about their historical past, without noticing that that nostalgia is produced by the aspect of a patina due to a chemical reaction. In a manner of speaking, a very famous patina that has amazed many is found covering the Statue of Liberty. I believe that few would believe today on seeing the green tone of this imposing figure, which when it arrived in New York was coppery brown.

Statue of Liberty and its characteristic green color resulting from the purple patina. Source: pxhere.com.
Well friends, until here the present post. I hope you have found the information shared interesting, and that if you are going to recreate the patina on a copper object you can tell me how it looks. See you next time!
References
- Wikipedia.com. Pátina (cobre)
- Wikipedia.com. Malaquita
- Wikipedia.com. Azurita.
- Wikipedia.com. Cardenillo