The stability of chemical substances
Well-developed analytical methods are available to prove the presence of a compound body and specify its composition. However, the compound that a scientist is interested in frequently only occurs in conjunction with other substances having a similar chemical composition, or else it is so unstable that it cannot be isolated. In this situation, identifying the compound body turns into a difficult challenge that can only be resolved using physico-chemical processes, such as the analysis of absorption spectra in the ultraviolet, visible, and infrared, the determination of vapor pressures, the study of magnetic properties, electrical conductivity, etc.
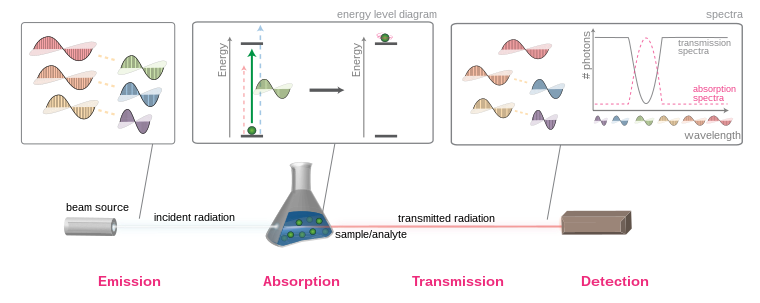
An overview of electromagnetic radiation absorption.
Numerous research by J. Gibbs, D. Mendeleev, and others on the issue of the relationship between a body's composition and its properties led to the development of physico-chemical analysis, which has a theoretical underpinning and a wealth of practical applications.
Let's say we mix two bodies A and B in a variety of proportions; we can get each mixture to have a specific value for some feature, like viscosity, by varying the amounts of each body in the mixture. The compositions of the various mixtures can be plotted along the abscissa axis, and the value of the property being measured can be plotted along the ordinate axis, to create a diagram. We shall see a "single point" on our diagram whose abscissa corresponds to the composite (Ax, By) if it forms between its elements for a specific fraction of the mixture.
When a system's state-determining parameters (p, T, etc.) are continuously varied, the properties of the phases that make up the system are likewise continuously varied, and each phase corresponds to the property diagram. composition a precise geometrical shape (a line or a curve for example). Many issues in metallurgy, mining, chemical technology, etc. will be resolved with the use of such graphics.
It is clear that the stability of these molecules is necessary for the potential of isolating a certain substance in its pure form or even confirming that it exists.
What factors affect chemical stability?
For instance, a mixture of hydrogen and oxygen is stable at high temperatures, whereas their combination, or water molecules, are stable at low temperatures. As a result, it is important to describe the conditions under which chemical systems can exist when examining their stability. Additionally, it is crucial to understand the exact nature of the compounds that the compound under investigation will decompose into. The stability may vary greatly depending on the manner of decomposition.
It's crucial to understand how the products of decomposition are accumulating and what kind of structure they have. We can see that under precisely identical circumstances, we can only rigorously compare the relative stabilities of compound substances.
References:
Les matériaux au cœur du processus d'innovation - N°59- Clefs CEA No 59 – Parution : Eté 2010
[Smail Meziane: Livre Chimie générale- Structure de la matiére. Berti edition, Alger, 2006]
[Livre- Chimie génerale- R.Ouahes]
Understanding that chemical stability actually starts with understanding the difference in chemical composition is highly essential even before we start the process of a chemical mix-up.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.