HTE, a highly promising method for producing hydrogen - Part2-
The electrochemical cell is one of the important components of the electrolyzer HTE, in which the dissolution of the water molecule occurs, this cell contains three ceramic layers (cathode, anode, and electrolyte). To ensure fluid distribution at the cell level, there are internal conductors to provide the electric current.
The HTE process's key characteristic is high temperature operation, which lowers the electrochemical overvoltage imposed at the electrodes. The HTE electrolyzer operates between 700 and 900 °C, which would have the benefit of requiring less specific electricity than electrolysis done in the traditional manner. The temperature of operation and the materials used for the Membrane Electrode Assembly (MEA) determine the current density that the HTE cell can handle.
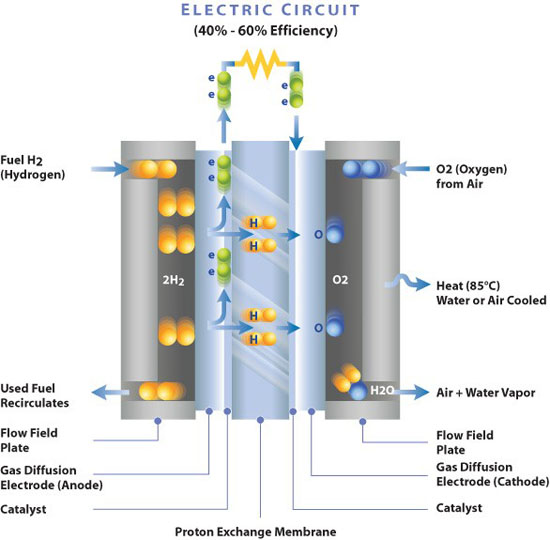
Membrane Electrode Assembly - Electro-Chemical Reaction Diagram- Wikipedia
With the exception of a few unique features, the restrictions imposed on the materials that make up this HTE electrolyser rarely differ from those that apply to SOFC fuel cells (Solid Oxide Fuel Cells).
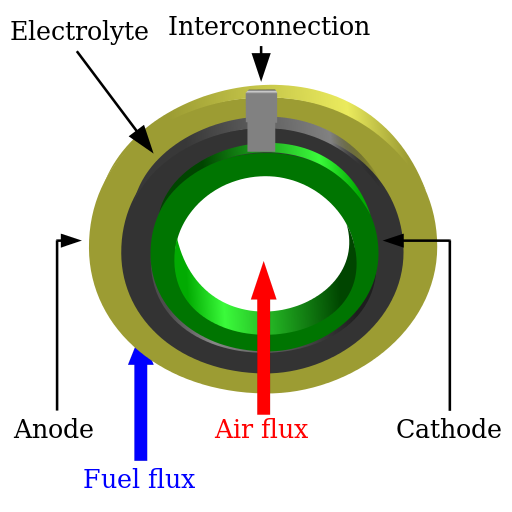
Schema of a solid oxide fuel cell (SOFC)
So, the electrolyte used in solid oxide fuel cells (SOFC), using Yttria-stabilized zirconia (YSZ), is similar to the one being investigated for this technology (HTE). On the cathode side, there is a mixture of water and hydrogen; it is necessary to separate the hydrogen from the leftover steam since some of the steam flow used to create hydrogen at the cathode is not dissociated, while on the anode side, there is oxygen O2, Currently, 850°C is the desired operating temperature for (O2-) conduction HTE cells.
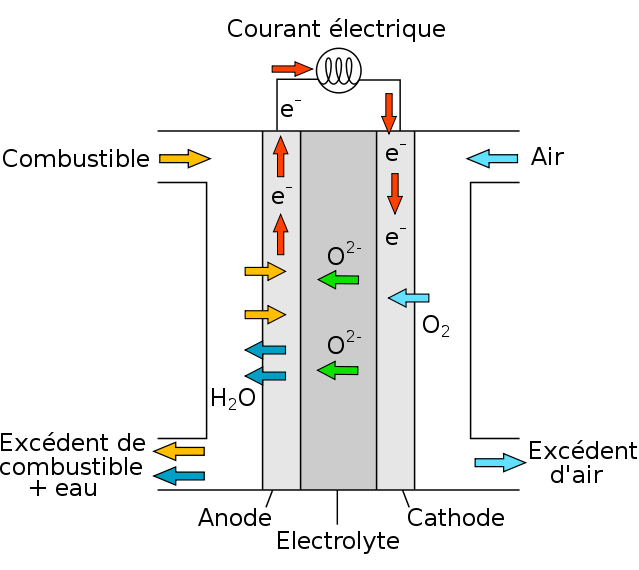
Scheme of a solid-oxide fuel cell (SOFC)
For a high-temperature water vapor electrolysis (HTE) system to be an economical option, it must remain inexpensive. Also, some technical challenges must be overcome, especially with regard to the interconnecting material, which must have a good thermal expansion capacity in order to protect the ceramic cells associated with it from rupture, and its electrical conductivity must be considered, and it must have a high resistance to oxidation, and it is important Also that does not interfere with the performance of electrochemical cells.
Electrochemical cell materials must have some properties, and it is necessary that all materials that make up these cells be of low price and abundant in order for the analysis process to be technically and economically feasible. The cell must possess enough mechanical resistance, particularly the ceramic layer, which serves as a mechanical support. The electronic conductivity of the material that is supposed to be used in the manufacture of the electrodes must have a value greater than 100 S/cm, and these electrodes must be characterized by high porosity in order to avoid the dismantling of the electrodes layers due to excessive local pressure, and also to facilitate the diffusion of water vapor.
As for the electrolyte-forming material, it must be non-porous and have a density greater than 95 percent, and it should not be affected by the reducing or oxidizing environment and maintain its stability in these two environments. Also, the coefficient of expansion of this material must be approximately equal to the coefficient of expansion of the electrodes. Finally, the ionic conductivity of this material at the operating temperature should be greater than 0.01 S/cm.
In essence, the electrolyte and cathode are both made of ceramic materials, while the cathode is made of cermet, a metal-ceramic composite.
According to the scientific journal Clefs, researchers in The Atomic Energy and Alternative Energies Commission (CEA) believe that one of the best ways to maintain operating systems is to reduce the temperature, and that, of course, without reducing performance. This method was chosen by the research team, in order to limit the degradation rate to 0.5% per 1000 hours of operation. The consistency of performance with durability is of great importance for the economic adoption of this technology.
References:
"Progress in SOFC technology development at fuel cell energy," in Proceedings of the 20th Annual Solid Oxide Fuel Cell (SOFC) Project Review Meeting, Washington, DC, April 30, 2019.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.