HTE, a highly promising method for producing hydrogen - Part5-
New materials incorporated into the HTE electrolyser cells:
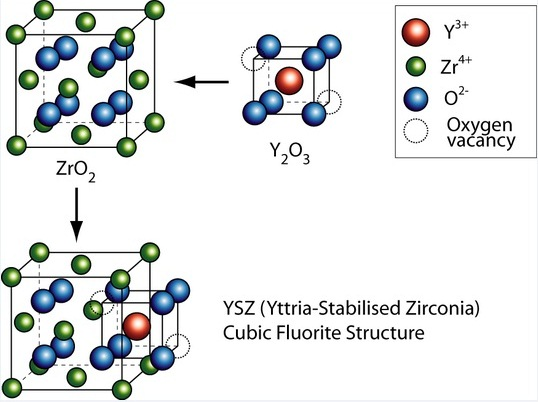
It is a nickel/zirconia cermet stabilized with yttrium oxide (Y2O3), (Ni-YSZ), with 8% yttrium oxide used as the hydrogen electrode in the HTE electrolyser cells; and a lanthanum manganite doped with strontium, designated LSM, for the oxygen electrode. And the electrolyte is made of yttria zirconia (YSZ). These substances are particularly well suited for use at 800 °C.
Ni and YSZ were mixed at a 40%vol% Ni concentration to create the anode material for cells. The cermet powder is compressed with the help of the surface activated sintering (SAS) process.
La1xSrxMnO3, where x is the doping degree, is the usual formula for lanthanum strontium manganite (LSM), an oxide ceramic element. It has the general form ABO3 and a perovskite crystal structure. Lanthanum and strontium atoms occupy the crystal's "A" positions, whereas smaller manganese atoms occupy the "B" places. To put it another way, the substance is composed of lanthanum manganite with strontium atoms replacing some of the lanthanum atoms.
New electrode and electrolyte materials, together with new configurations, are anticipated. Researchers are working to find ways to obtain good electrochemical performance at average temperatures between 650 and 750°C, and this, as we have said before, with the aim of compensating part of the electrical energy by heat to reduce costs.
Scandium oxide stabilized zirconia appears to be a superior candidate than yttria zirconia (YSZ) for innovative electrolyte materials suitable for intermediate temperatures due to its improved ionic conductivity. But, in addition to its relatively high cost, researchers are still studying its ability to stabilize and resist degradation.
In order to make the ionic and electronic conductivity good at medium temperatures, researchers seek to replace perovskite with another type of the same class, and it is related to mixed electronic and ionic conductive materials, such as strontium-doped lanthanum ferobaltite and lanthanum cobaltite, this change would reduce the importance of overvoltage for the inherent polarization at a temperature of less than 800°C with respect to the oxygen electrode.
Preliminary research indicates that a cell in which nickelate has substituted LSM develops improved electrolysis performance by a factor of 2 at 850°C and by a factor of 4 at 750°C. This work is being done by the Institute of Condensed Matter Chemistry in Bordeaux in partnership with EDF and the CEA.
Nickel is still the most powerful electrocatalyst for the hydrogen electrode that has been discovered so far. To increase conductivity at low temperatures, nickel/cerin materials doped with godolinium oxide (Ni-GDC) or samarium (Ni-SDC) are employed instead of standard nickel-zirconia yttria cermets.
- Researchers around the world are seeking to find ways to produce green hydrogen at the lowest possible cost and more efficiently. Where, by using ultraviolet light, Japanese scientists were able to efficiently separate water into hydrogen and oxygen. The important finding opens up new opportunities for energy production using aluminum-doped strontium titanate as a photocatalyst, whose properties have been extensively studied, according to Tsuyoshi Takata of the Shinshu University study team. The work was published in the journal "Nature" on May 27. He went on to clarify that in this case, the auxiliary catalysts—cobalt oxide for oxygen and rhodium for hydrogen—were carefully chosen and modified to guarantee that they only participated in the activities that were required.
- In the same context, an international team, consisting of chemist Ning Yan and his team at the Van te Hove Institute for Molecular Sciences at the University of Amsterdam along with colleagues from the faculty of Physics and Technology of Wuhan University in China, were able to harvest hydrogen from nano-sized gardens. In water splitting electrolysis studies, the researchers showed that their nano-gardens promoted not only hydrogen synthesis but also oxygen creation. This bifunctional activity was demonstrated in a symmetric dipole setup with exactly equal nanoparks at the anode and cathode.
Potential applications for "clean" hydrogen energy vary from power network control to automobile fuel cells, and the global market for hydrogen generation is expected to exceed 200 billions by 2030.
References:
"Progress in SOFC technology development at fuel cell energy," in Proceedings of the 20th Annual Solid Oxide Fuel Cell (SOFC) Project Review Meeting, Washington, DC, April 30, 2019.

Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.