HTE, a highly promising method for producing hydrogen
Most countries of the world seek to change their energy policy to reduce carbon dioxide emissions (pollution), that is, protect the environment and climate, in addition to obtaining permanent sources of energy. Hydrogen is an ideal source of clean energy, and it is used in many industrial fields and as a fuel in the field of transportation, and when burned, it doesn't release any damaging gases into the atmosphere, such as carbon dioxide. What makes this element very important as an alternative energy is its abundance, as it is the most abundant element in the universe.

Hydrogen recharging station in Japan- By Iwatani in Ariake
Hydrogen gas is often produced from the electrolysis of water, and the challenge here lies in the method of electrical power generation used in this process, as manufacturers and researchers seek to rely on renewable energy sources such as wind energy, as well as find new scientific methods to reduce the cost of production.
The production of hydrogen by electrolysis of high temperature water vapor "HTE":
One of the very important conditions for hydrogen production and reliance on it as an energy alternative is that the production process be highly efficient and low in cost. Conventional water electrolysis plants (electrolysis of water at ambient temperature) are able to achieve a total yield ranging from 25 to 28 percent. While the process of electrolysis of water vapor at high temperatures is more efficient (40 to 50 percent) depending on the main source of energy.
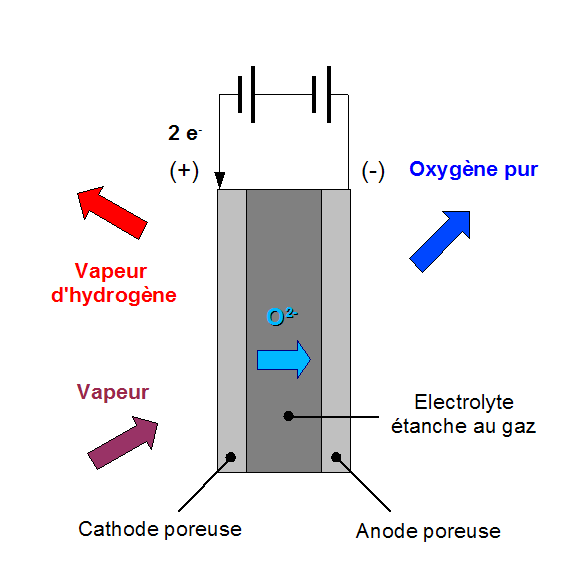
Diagram of high temperature electrolysis (HTE)- Drawn by Grimlock
Part of the energy that the water molecules need to break down will be provided by heat and thus the cost will be lower because heat is cheaper than electricity. In addition, the reaction efficiency is better at high temperatures, due to these very important factors. The electrolysis technique of water vapor is used at high temperatures ranging between 700 and 900 ° C to produce hydrogen from the dismantling of water molecules, with oxygen obtained as a byproduct of this reaction.
References:
"Progress in SOFC technology development at fuel cell energy," in Proceedings of the 20th Annual Solid Oxide Fuel Cell (SOFC) Project Review Meeting, Washington, DC, April 30, 2019.
This type of refining must be used in several industry
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Alternative energy source is something that we need to find soon
!1UP
You have received a 1UP from @gwajnberg!
@stem-curator, @vyb-curator, @pob-curator, @neoxag-curator, @pal-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.