Chemistry of water -Part 6-
Natural water:
Rainwater plays a significant role in nature because it removes gases like carbon dioxide, sulfur dioxide, and ammonia from the atmosphere as well as dust and other contaminants. And salts that are less soluble, such as gypsum CaSO4, dissolve when they come into contact with water on the ground, it also causes the dissolution of calcium carbonate as well:
CaCO3 + H2O + CO2 ==== Ca (HCO3)2
In addition to its dissolving effect, water also erodes rocks, carries soil, remains of animal and plant, and carries all this to seas and other bodies of water. This explains why the amount of dissolved salts in water varies according to its source, sea water contains about 3.5 percent of salt, and the rest of the water contains much less than this. However, the water from wells is the most salt-rich, followed by spring and river water.
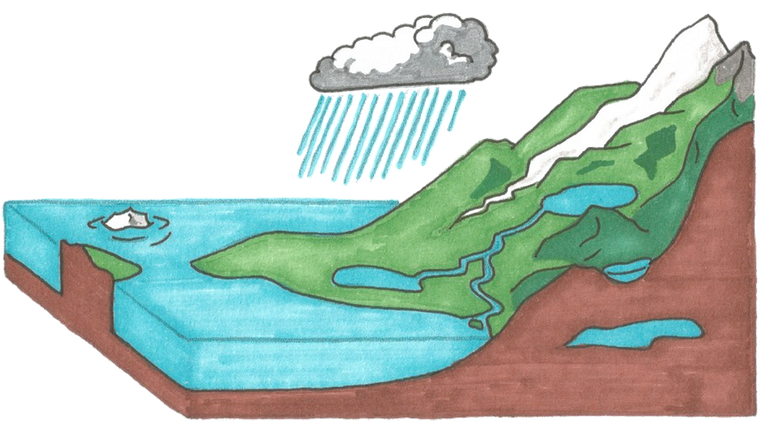
Natural water- Pixabay
Drinking water:
The spread of clean water networks in cities and the regularity of public sewage are the reasons that limit the spread of epidemics, as we no longer frequently hear about the spread of diseases like typhoid and cholera as we once did.
In some cities, it is possible to use pure water directly from a source. However, most cities have depleted nature of pure water sources, necessitating the use of various purification techniques to make their water pure.

[Pictures I took during a training visit to the water treatment plant in the city of Mostaganem - Algeria in 2020]
Boiling the water for a few minutes will kill the bacteria in it, but the process will leave the water tasting bad. In some cities, water is sterilized by ozonation, which kills the germs in it without altering the taste, and by adding chlorine to the water in a very small percentage, which also kills the germs in it. However, despite making the water safe from a health standpoint, these methods do not make the water free of suspended matter, since it must be filtered through large filters with layers of sand and gravel, it must first be treated with a small amount of iron sulfate and aluminum sulfate to create deposits that carry impurities.
Bibliographic references:
Kada, Imene- Double ionisation de la molécule d’eau par impact d’électrons- Phd thesis
[General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
Berend Ensing; geboren te Delfzijl- Chemistry in Water. Phd Thesis. University of Amsterdam- 2003
[AQUAPROX- Livre: Traitement des eaux de refroidissement. Imprimé en France par EMD S.A.S- 53110 Lassay-les-Chateaux. N° d'imprimeur: 15566- Dépot légal: juin 2006. N° 842- Cyclus print 90°]

Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.