ACUTE VIRAL HEPATITIS
ACUTE VIRAL HEPATITIS
Hello Hivers! Today's article is going to be on a fairly common condition that affects the liver- HEPATITIS. We will focus on the most common cause of HEPATITIS which is Viral Hepatitis. I really hope this educates you.
INTRODUCTION
Simply put Hepatitis is inflammation of the liver tissue. Hepatitis could either be temporary ie lasting for less than 6 months (acute) or long term ie lasting for more than 6 months (chronic). This article is on Acute hepatitis.
Acute viral hepatitis is a systemic infection that predominantly affects the liver most often caused by hepatotropic viruses (A, B,C D, E & G). Other viruses can also cause Hepatitis such as cytomegalovirus, herpes simplex, coxackievirus and adenovirus.
Acute hepatitis can be self- limiting (resolve on its own) especially when caused by hepatotropic viruses A & E or progress to chronic hepatitis, when caused by hepatotropic viruses C & B and rarely may result in acute liver failure. Eventually the chronic form may progress to a condition known as liver cirrhosis (scarring of the liver), liver failure, or liver cancer. However the acute form are usually asymptomatic.
HEPATITIS A (HAV)
HAV is a 28nm RNA-containing virus of the Picornaviridae family. Most cases have little or no symptoms. But when symptoms occur, the time between infection and development of symptoms (incubation period) is between two and six weeks (or 15-50days). A Key feature of HEV is that it is self-limiting.
Management of HAV is basically supportive.
Risk of fulminant hepatic failure (FHF) is very low (0.01-0.1%). There's a 1% risk of mortality in patients over 40 years.
Epidemiology of HAV
Globally, about 1.5 million symptomatic cases occur annually and about 114 million infections (symptomatic and asymptomatic). Most of these occur in areas with poor hygiene and a poor sanitation infrastructure.
Prevalence & incidence are directly related to socio-economic conditions.
Infections occur predominantly in childhood.
More than 90% of children in developing countries are infected and have life-long immunity.
Routes of Transmission
HAV is often transmitted by eating food or drinking water contaminated with infected feces. HAV is found in the faeces of individuals with acute HAV in the pre-symptomatic and early phases of the disease. HAV is spread by oral contact or with something that has been contaminated by fecal matter from an HAV-infected person.
Risk factors
~Children living in poor sanitation and low-hygenic areas.
~Children living in areas with high incidence of HAV such as developing countries.
~Day-care employees.
~Families of children in day care.
~Consumers of high risk food (e.g raw shellfish)
~People traveling to endemic areas.
~Those participating in anal sex.
Clinical Features
i) Jaundice
ii) Fatigue
iii) Abdominal pain
iv) Loss of appetite
v) Nausea
vi) Diarrhoea
vii) Fever
viii) Dark urine
Laboratory Diagnosis
HAV is reliably diagnosed by anti-HAV IgM.
Presence of anti-HAV IgG indicates a previous infection.
Management
There is no specific medication or treatment for hepatitis A. Recovery from symptoms can take weeks or even months.
Treatment is basically conservative and supportive and is aimed at maintaining comfort and adequate nutritional balance, including replacement of fluids lost from vomiting and diarrhea.
Hygiene is very important and hands should always be washed after bathroom use.
Oral contraceptives and Hormone Replacement Therapy should be stopped.
Alcohol consumption is not advised.
Contacts should be vaccinated.
Prevention
Prevention can be achieved by vaccination, good hygiene and proper sanitation.
Vaccination for pre-exposure prophylaxis (VAQTA or Havrix) provides long-term protection for up to 20 years.
HEPATITIS B (HBV)
HBV is a 42nm DNA-containing virus of the hepadnaviridae family.
It comprise of:
-inner core protein (HBcAg)
-outer surface coat (HBsAg)
It is present in most body fluids in infected individuals with acute or chronic hepatitis & inactive carriers.
It is transmitted parenterally such as sharing needles, sexual contacts
Incubation period is 40-160 days
Epidemiology
Globally, about 5 million cases of acute HBV occur annually.
HBV infection is a global health problem with 2 billion people infected worldwide
-360 million are chronically infected
-520, 000 die each year (50,000 of acute HBV and 470,000 of cirrhosis or cancer).
HBV is most prevalent in China, South-East Asia, sub-Saharan Africa, most Pacific Islands & the Amazon basin. It affects high risk individuals such as health care workers in developed countries and affects children in developing countries.
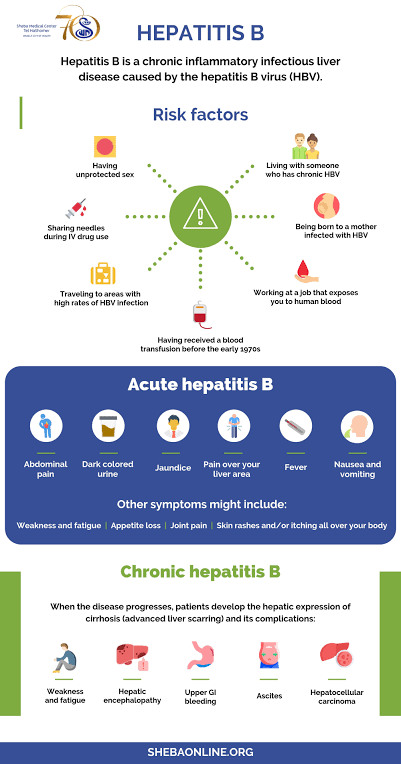
Risk factors
~Health-care workers
~Unsafe sex in any form
~Intravenous drug users and unsafe injection
~Haemodialysis patients (always become carriers)
~Unsafe blood transfusions
~Unsafe piercing, tatooing, acupuncture, tribal scarification, circumcision
~Migrant populations
~Asylum seekers & refugees
~Military personnel
~Tourists & students (unsafe sex)
~Firemen
Routes of Transmission
HBV is transmitted through body fluids such as blood, saliva & semen.
The route of transmission may be:
1.Vertical (from mother to baby at birth)
2.Horizontal (from child to child)
3.Parenteral (from unsafe injections & transfusions)
-Nonsterile instruments, tatoo needles, dental equipment, other sharp objects used for e.g scarification or female circumcision
4.Sexual contact (unprotected sex either homosexual or heterosexual)
Clinical Features
Principal symptoms
-Fatigue & tiredness
-Malaise
-Jaundice
-Fever
-Myalgia/athralgia
Less common symptoms
-Headache
-Nausea
-Diarrhoea
-Weight loss
-Abdominal discomfort
-Depression
-Anxiety
-Irritability
Laboratory Diagnosis
In a suspected case of acute infection, HBsAg will be positive & should have cleared within 3-6 months after the acute onset.
A follow-up re-check should always be carried out.
Management
Acute HBV infection often times does not require medications such as anti-viral treatment and most (95-99%) adults clear the infection spontaneously
Antiviral therapy is not likely to improve recovery rate & is not required in acute cases.
Prevention
Vaccination continues to be the best way for preventing this condition.
Two types of vaccines are available:
1.Recombinant or genetically engineered vaccines are made using HBsAg synthesized in yeast or mamallian cells into which the HBsAg gene has been inserted.
2.Human plasma-derived vaccines (PDVs) are prepared from purified HBsAg from the plasma of individuals with chronic HBV infection
HBV vaccines generate protective levels (>10 IU/mL) of antibodies to HBsAg in 95% of children & 90% of adults.
Revaccination is effective in 80% of those who do not respond to the primary vaccination
Types of vacinnation are pre-exposure & post- exposure
Contraindications to vaccines includes: fever >38.5, allergy to previous vaccines.
Outcomes of HBV
Outcome depends on immunological factors and age at which infection occurs.
HBV infection leads to one of four outcomes:
1.Recovery after acute infection (>95% in previously healthy adults over the age of 40)
2.Fulminant hepatitis
3.Chronic hepatitis B
4.Inactive carrier state
Hepatitis C (HCV)
HCV is an RNA-containing virus of the flaviviridae family. It's Incubation period is from 15-160 days.
Most infected cases of HCV both acute & chronic are asymptomatic, and if symptoms occur, they usually last from 2-12 weeks.
Epidemiology
As of 2015, about 143 million people worldwide were infected with hepatitis C. Incidence of symptomatic new infections is 1-3/100, 000. Most cases of HCV are seen in Africa and Central and East Asia. HCV infects only humans and chimpanzees.
Route of Transmission
HCV is mainly transmitted via blood-to-blood contact such as from an infected mother to her baby during child birth, intravenous drug use, non-sterilized medical equipment, needleprick injuries among healthcare workers, and blood transfusions.
Individuals at Risk
~Intravenous drug users
~Haemodialysis patients’
~Recipients of blood or solid organs
~Individuals with undiagnosed liver problem
~Infants born to infected mothers
~Healthcare workers
~Individuals with multiple sexual partners or having sex regularly with an infected partner
~Individuals with STDs
~Other potential risk activities include cocaine snorting, tattoos, body piercing, tribal scarification & circumcision ceremonies.
Clinical Features
Symptoms are generally mild and includes;
~decreased appetite
~fatigue
~nausea
~muscle or joint pains
~weight loss
Laboratory Diagnosis
1.HCV RNA can be detected in blood 1-3 weeks after exposure. PCR tests detect HCV RNA in serum within 1-2 weeks after infection
2.Antibodies to HCV (HCV-Ab)are detected by EIA (Elisa-Immunoglobin Assay) only in 50-70% when symptoms begin, with rate rising to more than 90% after 3 months. Antibody may be undetected for up to 8 weeks after infection (window period). Presence of antibody does not confer immunity
3.HCV core Ag testing: this detects HCV infection 1 and half months earlier than HCV-Ab screening test. It detects HCV infection an average of 2 days later than quantitative HCV RNA detection in specimens
4.Liver enzymes - ALT rises within 4-12 weeks.
Management of HCV
HCV causes chronic infection in nearly 80% of cases. So early identification of HCV is important because there is evidence that early intervention with standard interferon alpha can markedly reduce the risk of chronic infection from 80% to 10%
There is no pre-exposure prophylaxis for HCV
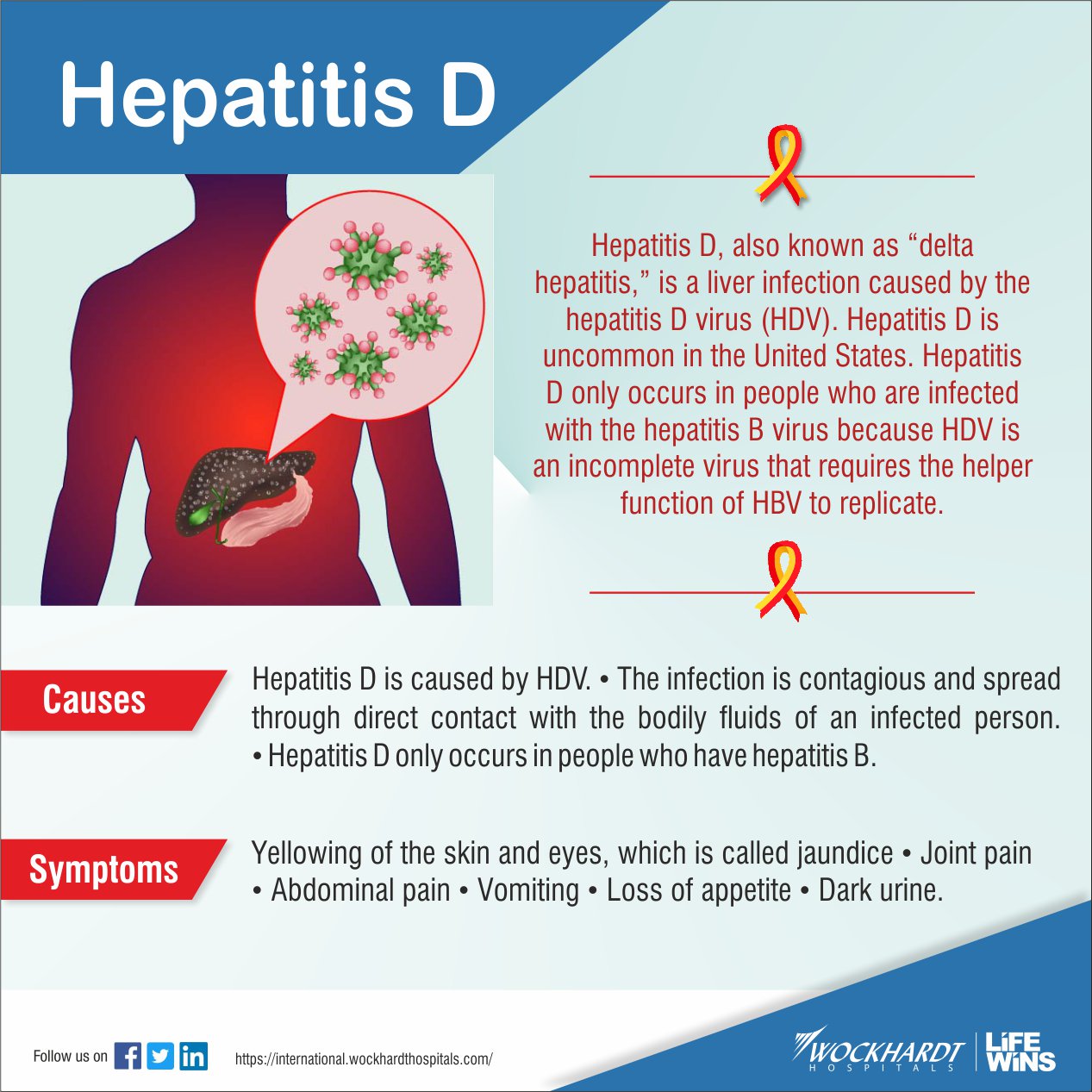
HEPATITIS D (HDV)
The HDV is a defective, small, spherical virus with a 36 nm diameter. It is a single stranded RNA virus of the delta viridae family. It is not a complete virus so it requires the hepatitis B surface antigen to transmit its genome from cell to cell. It therefore occurs either simultaneously with HBV (as a co-infection) or super-imposed with individuals with chronic form of HBV. It's incubation period is from 30-180 days. Due to its reliance on HBV, the duration of HDV is entirely determined by the duration of HBV infection.
Epidemiology of HDV
Globally, over 15 million people are co-infected. HDV is not so common developed countries, but it is common in regions such as the immediate Mediterranean region, sub-Saharan Africa, the Middle East, and the northern part of South America.
Outcomes of HDV
Co-infection
-severe acute disease
-low risk of chronic infection
-indistinguishable from acute HBV
Super-infection
-usually develops acute exacerbation of chronic hepatitis
-high risk of chronic liver disease
Routes of Transmission
1.Percutaneous exposure: Intravenous drug users
2.Permucosal contact
3.Sexual contact
The mode of transmission of HDV is similar to that of HBV
Laboratory Diagnosis
HDV Ag & HDV-RNA (PCR) appear early
Anti-HDV IgM antibodies appear later.
It may take 30-40 days after the first symptoms appear before anti-HDV can be detected.
Management
No specific treatment is currently available. Although some success has been reported with the antiviral agent - Foscarnet.
Prevention
HBV-HDV co-infection is prevented by vaccination against HBV.
Preventing HBV infection prevents HBV-HDV superinfection.
Education to reduce risk behaviours in individuals with chronic HBV infection.
HEPATITIS E (HEV)
HEV is an RNA-containing virus of the Caliciv-iridae family. Like HAV, it is self-limiting, therefore management should be supportive.
Hospital admission & medication are generally not necessary, except for pregnant women & those with background CLD
It's incubation period is 15-60 days, average 40 days.
Epidemiology of HEV
HEV has been implicated in large epidemics in Asia, Africa & Mexico
Young adults (aged 20-40) are affected most frequently.
Overall mortality rate is 1-3%
Mortality rate in pregnant women is 15-25%
Prognosis is generally good except in pregnant women.
Routes of Transmission
The main route hepatitis E virus is transmitted is through the fecal-oral route due to fecal contamination of drinking water.
Individuals at Risk
~Individuals living in poor hygienic conditions
~Individuals with infected or unsafe water supplies
~ingestion of undercooked or infected meat
~transfusion of infected blood products
~vertical transmission from a pregnant woman to her baby during child birth
Clinical Features
i)mild fever
ii)reduced appetite
iii)nausea and vomiting
iv)abdominal pain
v)itching (without skin lesions)
vi)skin rash
vii)joint pain
viii)jaundice
ix)dark urine and pale stools
x)slightly enlarged tender liver (hepatomegaly)
Laboratory Diagnosis
The main diagnosis of HEV infection is often based on the detection of specific IgM antibodies of the virus in the patient's blood.
Management of HEV
HEV infection is self limiting
There is no specific treatment capable of altering the course of acute hepatitis E. As the disease is usually self-limiting, hospitalization is generally not required. Most important is the avoidance of unnecessary medications. Acetaminophen/Paracetamol and medication against vomiting should not be given.
However, hospitalization is required for people with fulminant hepatitis, and should also be considered for symptomatic pregnant women.
Immunosuppressed people with chronic hepatitis E benefit from specific treatment using ribavirin, an antiviral drug. In some specific situations, interferon has also been used successfully
Prevention
The best way to prevent HEV infection is to avoid using and drinking untreated and unsafe water.
Also eating of undercooked meat and uncooked shellfish and foods should be avoided.
Currently, there is no known vaccine for HEV.
HEPATITIS G (HGV)
Not much is know about this form of hepatitis, however HGV is a flavivirus that is percutaneously transmitted. It is associated with chronic vireamia lasting 10 years. It does not appear to cause important liver disease.
It also does not affect the response of patients with chronic HBV or HCV to antiviral therapy.
HGV co-infection may improve survival in patients with HIV infection.
Epidemiology of HGV
It has been detected in:
-1.5% of blood donors
-15% of chronic HBV or HCV
-20% of hemophiliacs
-30% of haemodialysis patients
-50% of IV drug users
REFERENCES
1.Matheny, SC; Kingery, JE (1 December 2012). "Hepatitis A". Am Fam Physician. 86 (11): 1027–34; quiz 1010–1012. PMID 23198670. Archived from the original on 9 March 2014.
2.Connor BA (2005). "Hepatitis A vaccine in the last-minute traveler". Am. J. Med. 118 (Suppl 10A): 58S–62S. doi:10.1016/j.amjmed.2005.07.018. PMID 16271543.
3.Ryan KJ, Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 541–4. ISBN 0-8385-8529-9.
.jpeg)
.jpeg)
.jpeg)
Congratulations @avose! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s) :
You can view your badges on your board And compare to others on the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Do not miss the last post from @hivebuzz: