THE JOURNEY TO MICROBIOME-BASED THERAPIES
By DataBase Center for Life Science (DBCLS) CC BY 4.0, Wikimedia
These microorganisms are in close interaction with the human body and their activities and composition affect the body's response to diseases, food digestion, nutrient utilization, response to cancer immunotherapies as well as brain functions and neural development. The understanding of the effect of this close interaction on human health has spurred scientists and researchers to develop therapies targeting these microbes in the treatment and prevention of diseases.
While the earlier understanding of these microbes was their disease-causing abilities, which led to the development of subtractive therapy (antibiotics), later studies and a better understanding of the microbes have also shown that they also contribute to the health of the individual and more studies are ongoing on how this positive effect can be harnessed to treat diseases.
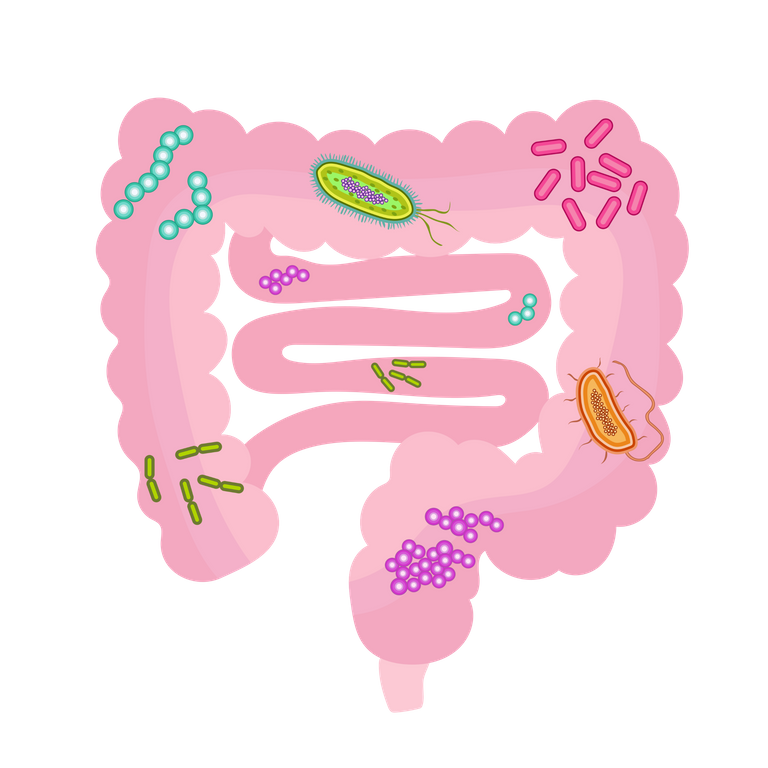
Of importance in this discussion are the microbes in the gastrointestinal tract called the gut microbes which have been found to not only affect the gut but also other organs like the heart, the liver, the bones, the brain, etc, and contribute to their health and diseases. The gut harbors the largest number and diversity of microbes, of which the majority of them are obligate anaerobic bacteria. Obligate anaerobes are organisms that cannot survive in the normal atmospheric oxygen concentration.
By Rachelshoemaker - Own work, CC BY-SA 4.0, Wikimedia
Through gene sequencing technologies and multi-omics profiling studies, scientists over the years have sought to understand these microbes, differentiate the important ones from the harmful ones, and study the types and effects of the substances produced by these microbes as well as the composition and functions of these microbes which enhance their effects on the immunity and metabolic processes in human.
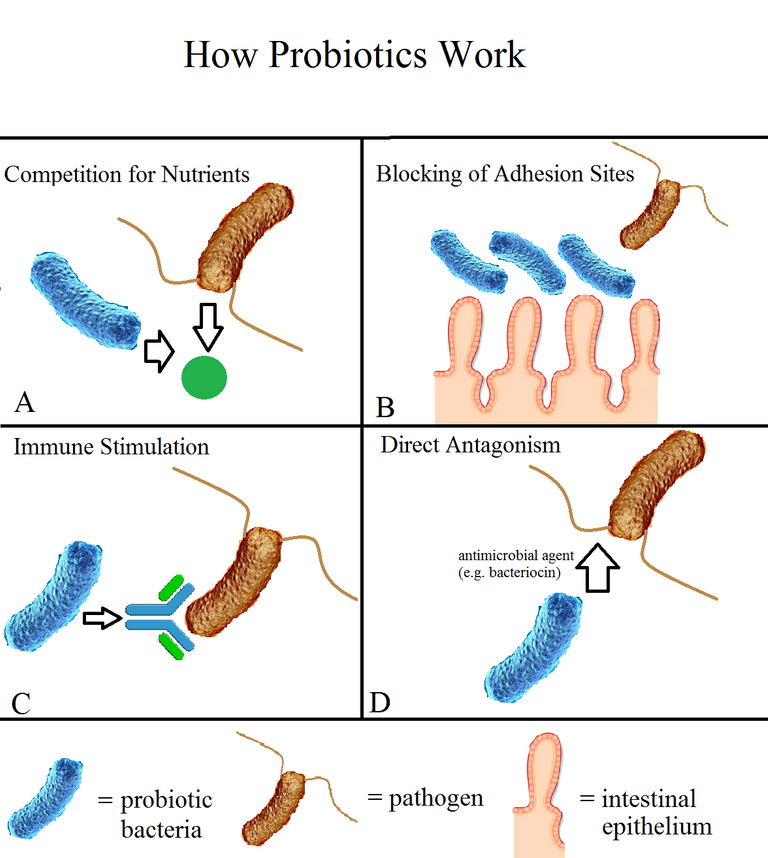
The manipulation of these microbes can improve human health through either additive therapy where the beneficial microbes are supplemented to the host microbiome to treat or prevent diseases, Subtractive therapy where the harmful microbes are eliminated from the host's microbiome (antibiotics), or Modulatory therapy where some agents are added to alter the activities or compositions of the host's microbiome.
By Wzhou8313 - Own work, CC BY-SA 4.0, Wikimedia
The knowledge of this gut microbiome has evolved into tremendous research on its application in the diagnosis and treatment of diseases. The basis of research in this field is trying to understand how these gut microbes are associated or correlated to the health and diseases in the human body. And most importantly, their function and the mechanisms through which they contribute to the body's health and diseases. Some success has been recorded and some microbiome-based therapeutics are already in use like Acidophilus Probiotics, Florastor, etc.
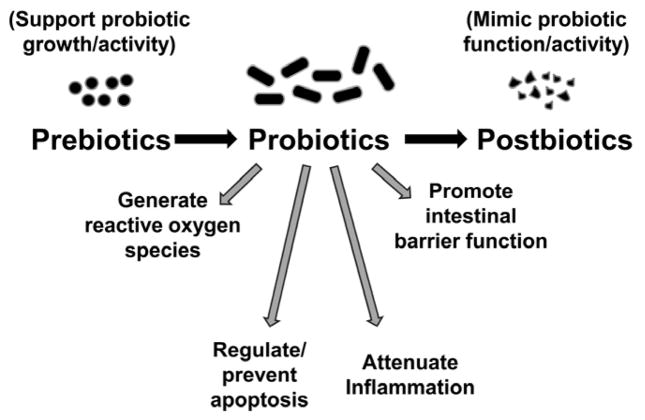
Many microbiome-targeted therapies such as dietary intervention, nutritional supplements, antibiotics, probiotics, prebiotics, synbiotics, postbiotics, psychobiotics, bacteriophage, and fecal microbiota transplantation have been used to regulate the human microbiome. So far, great progress has been achieved in treating metabolic diseases, cardiovascular disorders, and cancer.
However, this research is not without challenges.
One of the major challenges is that gut microbes are almost unique to every individual. The composition and activities of these microbes vary in each individual according to geographical location, sex, age, diet, drugs, and even lifestyle. So it has been a great challenge, trying to isolate and define the healthy microbes that are universal to every individual across a wide geographical location. So this makes it difficult to bioengineer particular microbes for global use.
Also, The safety and side effects of these therapies are still areas of concern as well as the mechanism of action and a standardized microbiome for analyzing and sequencing the microbes.
By Ravi Mangal Patel, MD and Patricia Wei Denning, MD, CC BY-SA 4.0, Wikimedia
As a step in the right direction in addressing these challenges, scientists at Stanford University yesterday, September 7, 2022, developed the most complex, complete synthetic microbiome.
Stanford University researchers have built the most complex and well-defined synthetic microbiome, creating a community of over 100 bacterial species that were successfully transplanted into mice. The ability to add, remove, and edit individual species will allow scientists to better understand the links between the microbiome and health, and eventually develop first-in-class microbiome therapies.
Most of the research works done before this new milestone used fecal transplants. In a fecal transplant, the feces of a healthy donor is introduced into a patient's gut, thereby transferring the entire natural microbiome of the donor into the recipient. This is done to restore the normal bacteria flora in the gut of the recipient.
However, this method didn't give them the tool to remove or edit individual microbiome species. But with this synthetic microbiome, they can now add, remove or edit individual microbiome species. This will greatly enhance a better understanding of these microbes and how they can be harnessed to develop better and novel therapies for man.
In summary, the indebt knowledge of these gut microbes will help us to develop new microbiome-based therapy as well as a tool for early diagnosis, monitoring the prognosis of patients, and conducting risk assessments on healthy individuals.
The research in this field is gaining more momentum by the day. Some microbiome-based drugs are already used in the clinic currently, but there is still much to know in this green field. And I am optimistic that with better knowledge and understanding of this gut microbiome, more life-changing drugs will be developed in a few years to come.
References and Further reading;
Congratulations @alidickson! You have completed the following achievement on the Hive blockchain and have been rewarded with new badge(s):
Your next target is to reach 600 comments.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out the last post from @hivebuzz:
Many thanks @hivebuzz . I appreciate your great support
YEah you are right! the mostly we know, more questions appear about our gut microbiome, maybe the cure of many diseases are there!

!1UP
Thank you so much. That's very correct.
The gut microbiome holds a bright future for better and more effective treatment of diseases.
You have received a 1UP from @gwajnberg!
@stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
Thank you so much @curation-cartel for your support. I am so very grateful.
It is really cool to read about these news. It is quite exciting for the future, and I am always amazed by what we can do in our labs.
Cheers!
Thank you Sir.
The future is indeed bright. With research, the hope of a better life with safer and more effective treatment of diseases is very glaring.
I fully agree on this!
Cheers!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Thank you so much @stemsocial . I appreciate
Hippocrates said that all disease begins in the gut. Autoimmune diseases will probably be solved by a gastroenterologist rather than an autoimmunologist.
That's very correct Sir. There is indeed a great future in the diagnosis and treatment of diseases through the gut microbiome.