Approach to the photoelectric effect
Introduction
In previous publications I shared a fascinating content in the field of science in the world of physics, where the phenomenon of light in order to understand, how light behaves if we consider it simply as a wave or light ray, also that it is part of an electromagnetic wave, which allows us to observe other physical phenomena, such as a longitudinal wave, this is the color wave if it is shown to be darker or lighter, which will depend on the magnitude of the disturbance, this time it will be shown from another point of view such that it is the photoelectric effect, where certain materials or bodies when exposed to light have the property or ability to absolve this light energy by emitting protons and stimulating photons as an energy source .
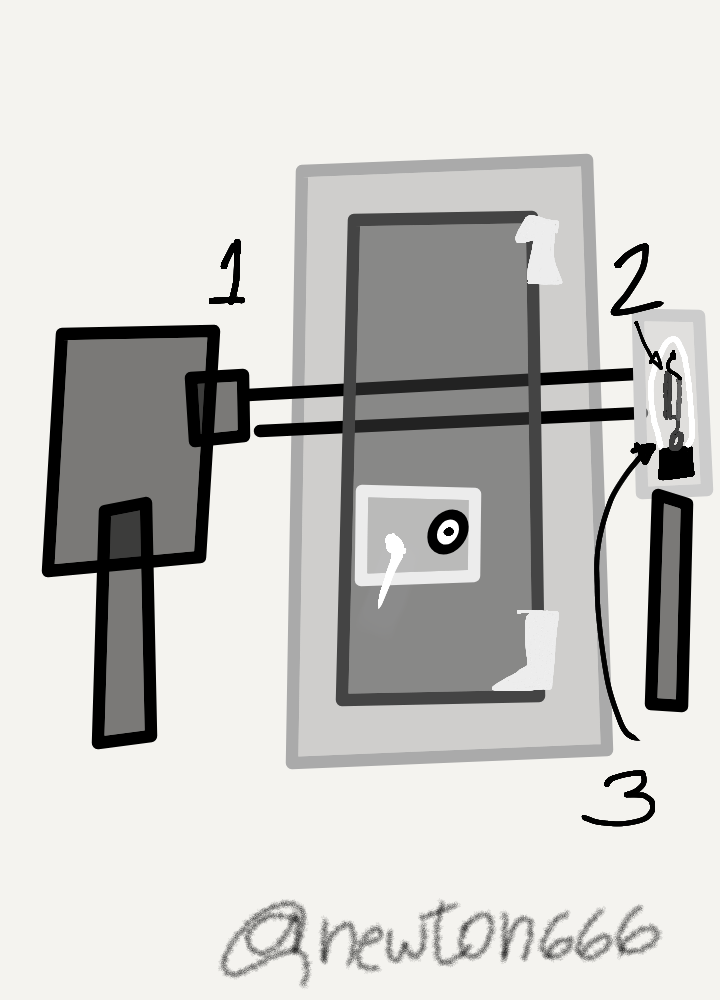
A representation of Photoelectric cell where "1" is the light source, "2" is the cathode and "3" is the anode.
The photoelectric effect
Considering that body or matter with a metal composition has been evidenced, when exposed to light it can store part of this energy, by means of the excitation of the electrons, then this energy can be expelled, which is why it consists in the emission of electrons through a material when it is hit by electromagnetic radiation, at the different frequency of light emission or also in the form of propagation in which visible light corresponds, to the human eye, ultraviolet.
The photoelectric effect: it is the emission of electrons from a metal exposed to light, the metal surface absorbs enough energy from light that some of its electrons are released from their atoms and escape outside from the surface. Information consulted in All about Einstein, 2005.
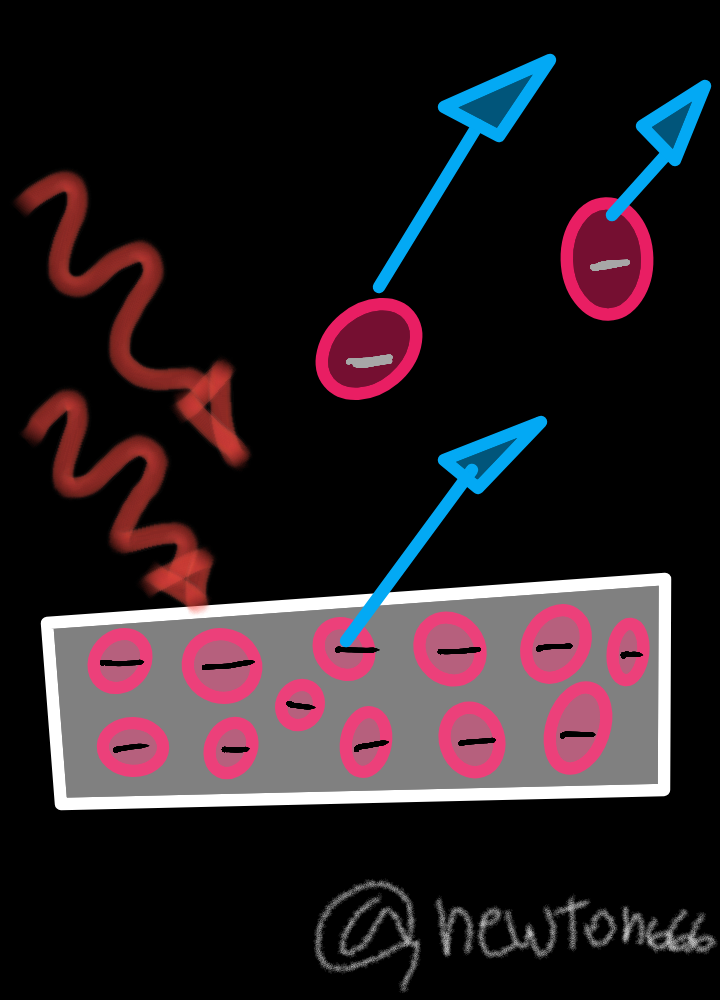
A representation of the diagram illustrating the emission of electrons from a metal plate, requiring the energy that is absorbed from a photon. It is also about understanding the behavior of photons, since they have a characteristic energy determined by the wave frequency of light, in which only an atom absorbs energy from a single photon, it has more energy than the initial one to expel an electron from the material.
What makes this physical phenomenon unique is that clairvoyance of other phenomena in which in a scientific test such as photoconductivity refers to the increase in the electrical conductivity of matter or in diodes caused by light, which allowed another study to be able to create solar cells to demonstrate the photovoltaic effect, which consists in the partial transformation of light energy into electrical energy, making it clear, dear reader, all this information on the photoelectric effect indicates that photons can transfer energy to electrons, these electrons do not escape from the metal at normal temperatures because they do not have enough energy, allowed great scientists to differentiate it from the common "X" rays, since only these "X" rays, the transformation into a photon of all or part of the energy was evident kinetics of a moving electron, over time was a support in the field of medicine. Another important fact is the following, the fact that the emission of electrons only occurs above a certain frequency, since it will depend on the radiation threshold, light is quantified regardless of its intensity, thanks to them it will allow us to learn some concepts , which will help us to explain how light can be created artificially and also to understand how the Sun is the greatest source of energy in the solar system.
Los principios básicos que rigen el efecto fotoeléctrico son los siguientes:
[1]-No hay emisión de electrones si la frecuencia de la luz incidentes cae por debajo de la frecuencia umbral y0, que es una característica del metal iluminado.
[2]-El efecto se observa si la frecuencia de la luz excede la frecuencia umbral, y el número de fotoelectrones emitidos, es proporcional a la intensidad de la luz; sin embargo la energía cinética máxima de los fotoelectrones, es independientes de la intensidad de la luz, lo cual es posible explicar con los conceptos de la física clásica.
[3]- La energía cinética máxima de los fotoelectrones se incrementa con el aumento de la frecuencia de la luz.
[4]-Los electrones de la superficie se emiten casi de manera instantánea, incluso a bajas intensidades. Desde el punto de vista clásico se esperaría que los electrones requieran algún tiempo, para absorber la radiación incidente, ante de que alcancen la energía cinética necesaria que le permita escapar de la superficie del metal. Información consultada en Física Moderna Edición Revisada por Norma Esthela Flores Moreno, Jorge Enrique Figueroa Martínez, 2007.
It is important to know and know how to handle this information, since light has many aspects or focus of studies at the level of science, as in the case of the behavior of light in phenomena such as the photoelectric effect, Albert Einstein resigned aside that light it is a wave and the hypothesis in it was a way for the possible explanation that light is made up of small “envelopes” referring to photons, which can only contain a certain amount of energy equal to a multiple of a fundamental minimum value; making it possible for the amount of energy to be discrete.
Photons: E = h.f
E is the energy, f is the frequency of light in general from electromagnetic radiation and h is a constant called Planck's constant, which has the following value:
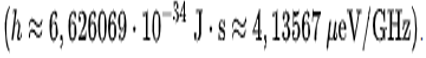
Conclusion
Thanks to all the information obtained and consulted, it allows us to understand another study approach on the phenomenon of light, such as the electric photo, which is evident if the radiation photons that hit the metal have a lower energy than that of work function, the electrons of the material do not obtain enough energy to emit from the metal surface, in another order idea we can also mention thanks to these contributions artificial light can be produced and application of technique for the field of medicine such as rays "X" ultraviolet stripes, ultrasound.

[1]- All about Einstein, 2005.
[2]- Modern Physics Revised Edition by Norma Esthela Flores Moreno, Jorge Enrique Figueroa Martínez, 2007.
