How Acid Rain is affecting our ecosystem and how to prevent it?
Acid rain is defined as the precipitation or rain that is usually acidic. It has PH value less than that of natural rainwater (which is around 5.6 due to dissolved carbon dioxide). In the atmosphere, sulphur dioxide and nitrogen oxide combine with water vapour and precipitate as sulphuric acid or nitric acid in rain, fog or snow. Acid rain is usually measured using pH scale. The lower a substance’s pH, the higher acidic it will be.

Causes of Acid Rain
There are both natural and human causes of acid rain. The natural causes include emissions from volcanoes and from biological processes which occur on land, wetlands and in oceans. There are also effects of acidic deposits which have been detected in glacial ice thousands of year old in remote parts of the world.
The main cause of acid rain is from human sources. These sources include industrial factories, power generating plants and vehicles. During combustion, sulphur dioxide and oxides of nitrogen are released which are main causes of acid rain.
Formation of Acid Rain
During rainfall or when water vapour condenses, they dissolve in the water to form sulphuric acid (H2SO4) and nitric acid (HNO3). In this way air is cleaned of the pollutants, but it also causes precipitation to become acidic.
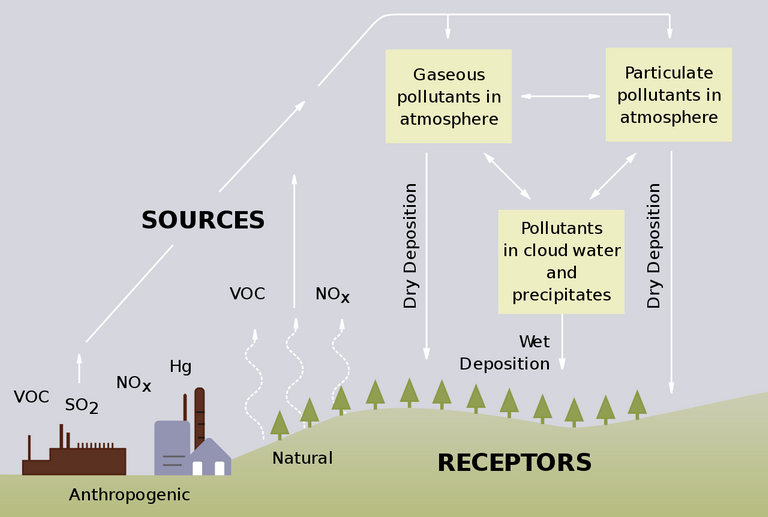
Acid rain can be in the form of wet deposition as well as dry deposition. In wet deposition, acid chemicals are blown into areas where weather is wet. The acid falls to the ground in the form of rain, fog, snow and mist. It affects wide variety of plants and animals as the water flows through the ground. In dry weather regions, the acid is incorporated into dust or smoke and then falls to the ground through dry deposition. It sticks to the ground, buildings, homes, cars and even trees.
Acid rain has badly affected some countries. Acid rain is main problem in Canada. Water and soil system don’t have natural alkalinity such as lime base, thus acid can’t be neutralized. Canada has vulnerable hard rocks such as granite which don’t have capability to neutralize effectively acid rain.
Industrial acid rain is also problem in China, Eastern Europe and Russia. In United States, acid rain from power plants has badly harmed the forests of upstate New York and New England. It means that effects of acid rain can spread over large area, far from source of the pollution.
Effects of Acid Rain
There are many harmful effects of acid rain on humans, animals, plants and infrastructure. Some of them are summarized below:
1. Acid rain is harmful for human health. It creates respiratory problems like dry cough, asthma, headache and throat irritation. The toxins from the soil can be absorbed by animals and plants, which indirectly affects humans severely. Severe exposure of acid rain may lead to brain damage, kidney problems and Alzheimer’s disease.
2. It is harmful for aquatic life. It increases acidity in water bodies which stops hatching of eggs of certain organisms and thus aquatic ecosystem is affected.
3. It is also harmful to vegetation. Increased acidity in soil can leach nutrients from soil which slows plant growth. It produces brown spots in leaves of trees which hinders photosynthesis process.
4. It accelerates the weathering process in stone structures and metals. i.e. Parthenon in Athens; Taj Mahal in India are affected by weathering process.

5. Acid rain can damage historic monuments, buildings and statues, especially made of rocks and contain huge amount of calcium carbonate. Acids from acid rain react with calcium carbonate to create gypsum which then flakes off.
Preventive measures against Acid Rain
We can take following precautionary measures to minimize the effects of acid rain:
1. We should try to reduce mount of sulphur dioxide and nitrogen oxides into atmosphere. This can be achieved by burning less fuel and using cleaner fuels and using natural gas.
2. We should use alternate sources of electricity like nuclear power, hydro-electricity, wind energy and solar energy.
3. In Norway and Sweden, liming is used as short term remedy to acid rain. Powdered limestone is added to water and soil to neutralize the effects of acid.
4. Flue gas desulfurization (FGD) is used in many coal burning power plants to remove sulfur containing gases. FGD can remove around 95% of SO2 in flue gases.
Thank you for reading! Stay Safe!👋😌
References:
0
0
0.000
I don't really understand this. but it is very useful.