Antibiotic Resistance Genes
In the process of gene transfer to plants, the special DNA piece to be transferred is cut from the DNA of the living thing with special DNA-cutting enzymes and added to a carrier DNA molecule, usually called a vector.
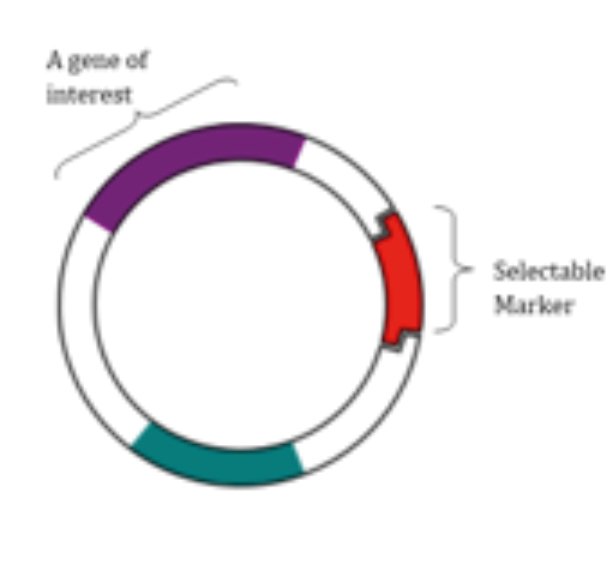
In addition to the DNA region to be transferred on the vector, there are antibiotic resistance genes that will enable the selection of individuals who receive the transferred gene and some special gene regions that will enable the transferred DNA to become functional.
The most popular antibiotic resistance genes are:
Neomycin phosphotransferase II (nptII)
Hygromycin phosphotransferase (hpt).
Other antibiotic resistance genes are:
Gentamycin acetyltransferase (accC3)
Bleomycin
Phleomycin
The NPTII enzyme transforms the effect of aminoglycoside antibiotics such as kanamycin and neomycin into inactive by providing phosphorylation in gene transfers to plant species. Hygromycin phosphotransferase is a suitable marker for both plant and animal systems, and the HPT enzyme makes the hygromycin B antibiotic inactive. Hygromycin is generally more toxic than kanamycin and causes sensitive cells to die more quickly.
Paul Berg created the first genetically modified DNA molecule in America in 1972. One year later; Stanley Cohen, Annie Chang and Herbert Boyer transferred an antibiotic resistance gene to the bacteria. If the first transgenic plant; In 1982, it became a tobacco plant with the gene for antibiotic resistance [4].
Marker genes are needed in order to select the cells that are transferred from Agrobacterium tumefaciens and in direct gene transfer methods and the plants that grow from these cells. Lateral transfer of antibiotic resistance genes in the microflora of the human digestive system is thought to threaten human health.
Another concern is that genes such as neomycin phosphotransferase II may be allergic [1].
The possibility of these genes used in transgenic plant production to spread to nature is seen as a great danger by some circles. It is said that if antibiotic resistance genes pass to pathogenic microorganisms, it will be very difficult to control the infections caused by these bacteria.
There are also debates arguing that it may lead to negativities such as playing a role in cancer development [2].
It is thought that antibiotic resistance genes may pass on bacteria in humans and animals, causing them to become resistant to antibiotics. However, this has not been proven experimentally [5].
In the meetings held by the World Health Organization (WHO), the United Nations Food and Agriculture Organization (FAO) and The European Food Safety Authority (EFSA), the probability of these risks was discussed and, albeit low, It has been reported that risks may occur. As a solution to this situation, it has been recommended to develop the following alternative methods [3].
Horizontal transfer of antibiotic resistance genes, risk perception of societies and consumer acceptance of products on the market, etc. issues have forced scientists to consider alternative methods.
Two general strategies are followed to avoid the use of antibiotic resistance genes:
Use of multiple selectable non-toxic marker genes (positive selection). A selectable marker gene, a DNA sequence, is used specifically to identify transformed cells. Plant transformation based on GFP (Green Fluorescence Protein) and mannose (Man) selection can be used as an alternative to antibiotic resistant marker genes. However, the number of multiple selectable markers is limited. However, it is an area open to improvement.
Elimination of the selectable marker gene in the obtained transgenic plant.
As a result; There are solutions such as the use of alternative genes, intensive and comprehensive laboratory and clinical tests before commercial cultivation of the products in question is allowed. The Biosafety Board should become the driving force for detailed studies on the subject and the society should be informed. A very serious task falls on the risk assessment committee. Although the Biosafety Law allows the importation of some of these products, the production of these products is not yet free in our country. This does not mean that worries are meaningless. It is a fact that intensive and sincere studies are urgently needed.
Posted Using LeoFinance Beta