Science contained in a kitchen fire
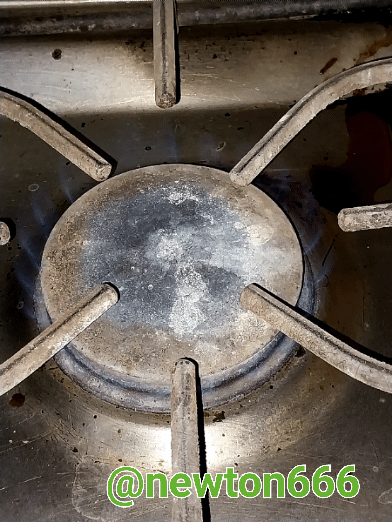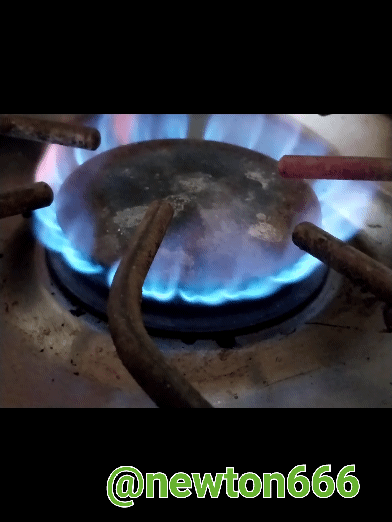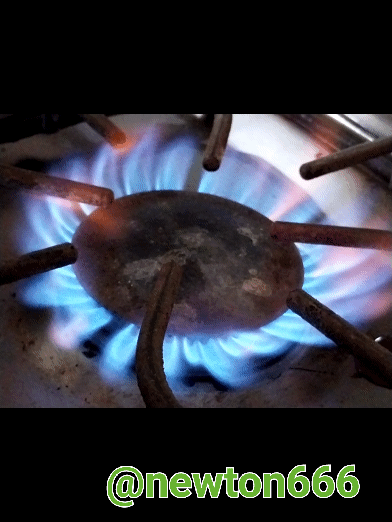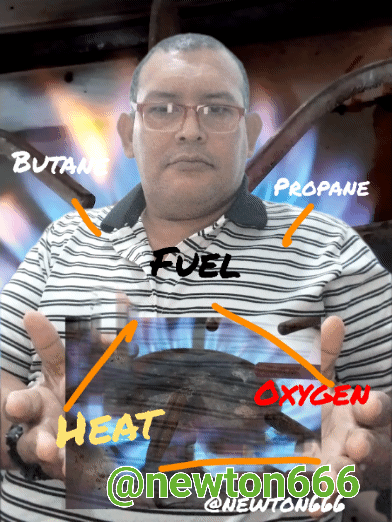


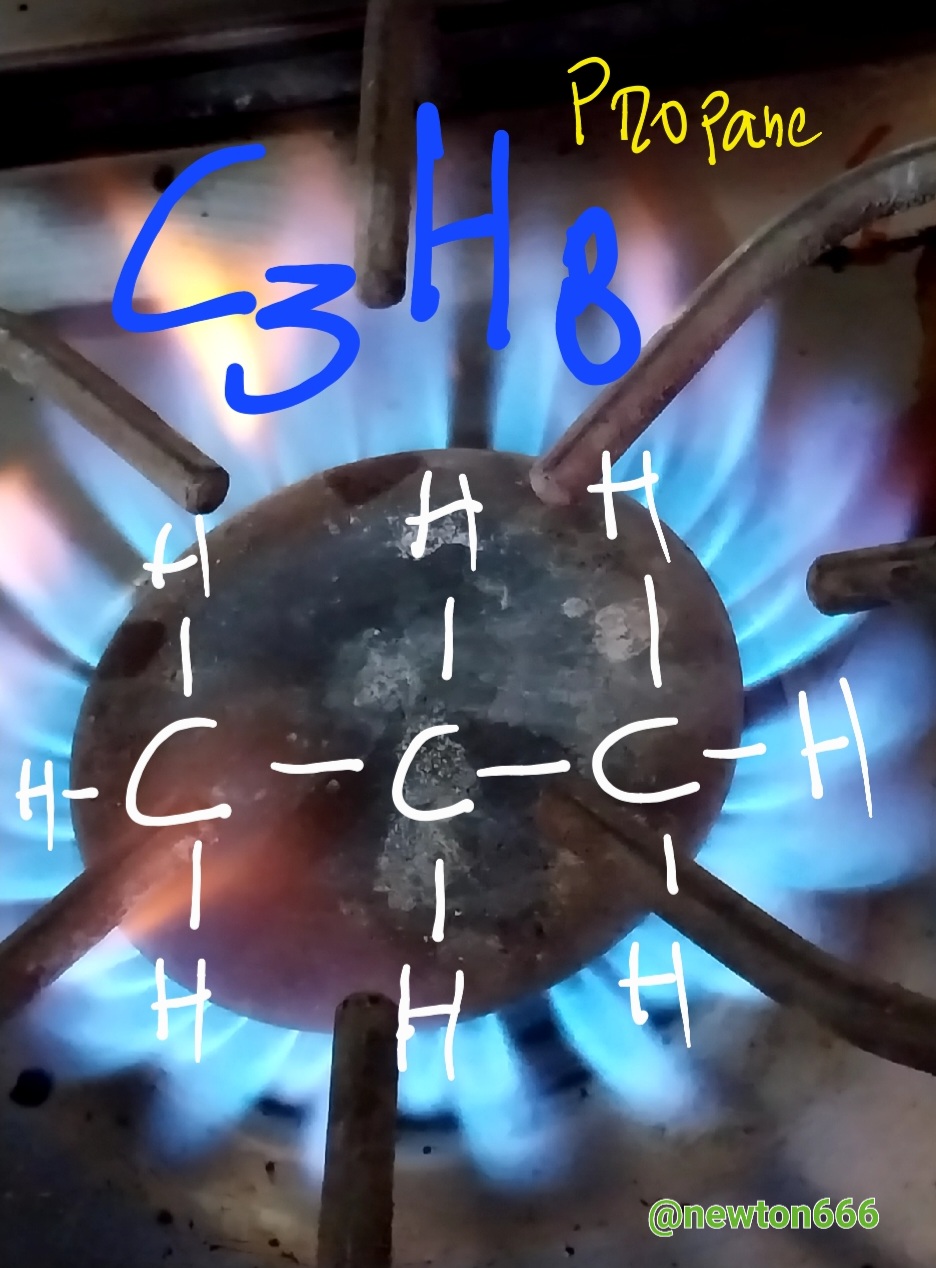
The good thing about science is that we see it every day in our daily lives, in my case I used the kitchen and why the fire is blue or what causes this phenomenon, well considering how propane and butane are gases of domestic consumption and each one of them has a different composition or formula that differentiates them, they are fuel as an energy source that maintains the balance of the environment, in the case of propane, dear reader, the singularity of this component is that it makes it particular, it belongs to aliphatic hydrocarbons with simple carbon bonds, known as alkanes, their composition is C3H8, what is fascinating is how with three carbon particles it is linked with eight hydrogen particles, its very particular characteristic that makes it stand out are its own physical properties It is Colorless in appearance, Density 1.83 kg/m³; 0.00183 g/cm³, Molar mass 44 g/mol Melting point 85.5 K (−188 °C), Boiling point 231.05 K (−42 °C). Many people wonder why the flame is blue, that is thanks to the combination of propane with oxygen, making a balanced reaction possible, while what happens with the flame, which is more of a reddish-yellow color, is an unstable reaction and you have to be careful not to danger.
A curious note we have known this gas since 1857 by the French chemist Marcellin Berthelot, thanks to his contributions another energy industry was born as part of an industrial revolution, since we must be aware that it is a by-product of the processing of natural gas and of the oil refining, well today this source of energy is still maintained.

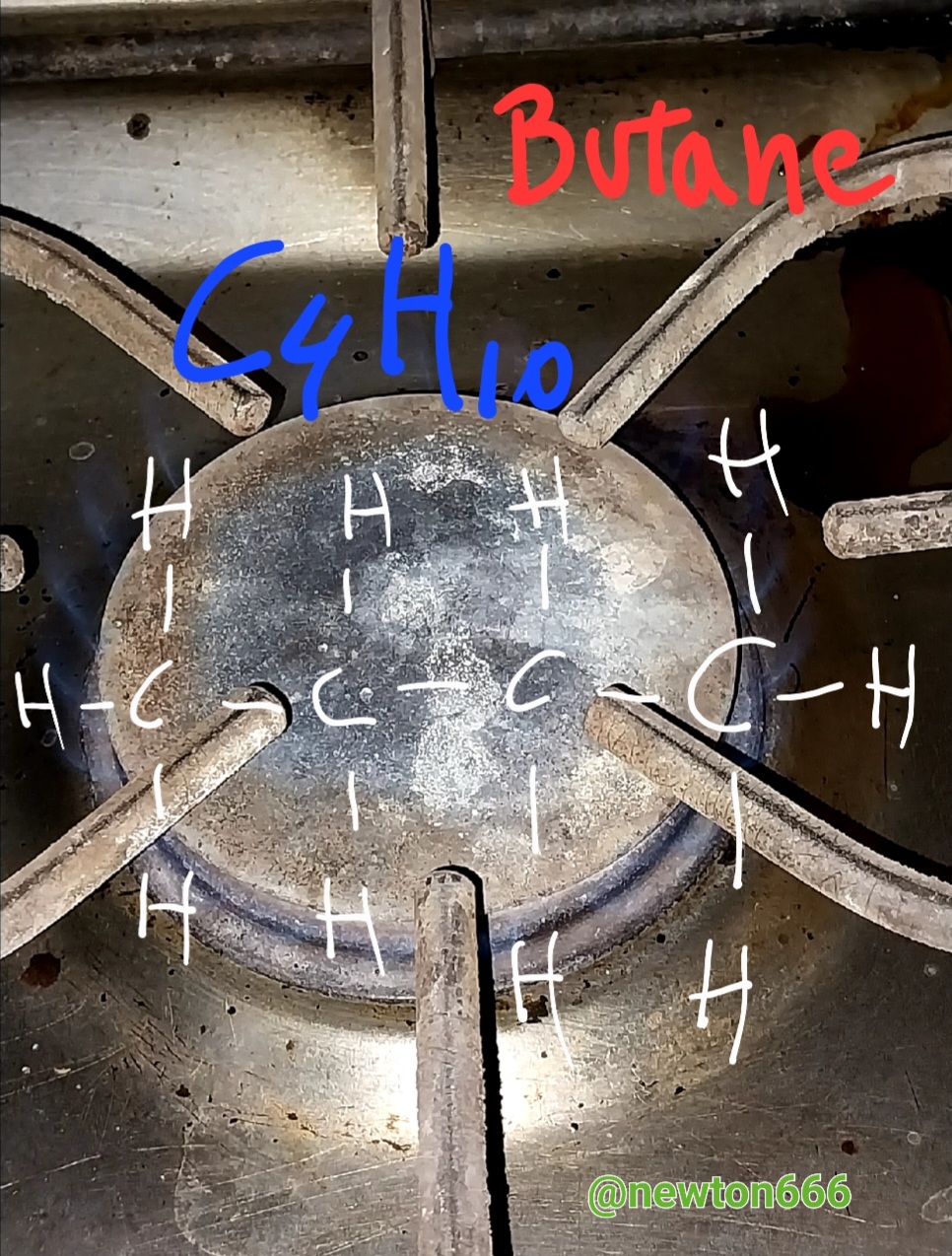
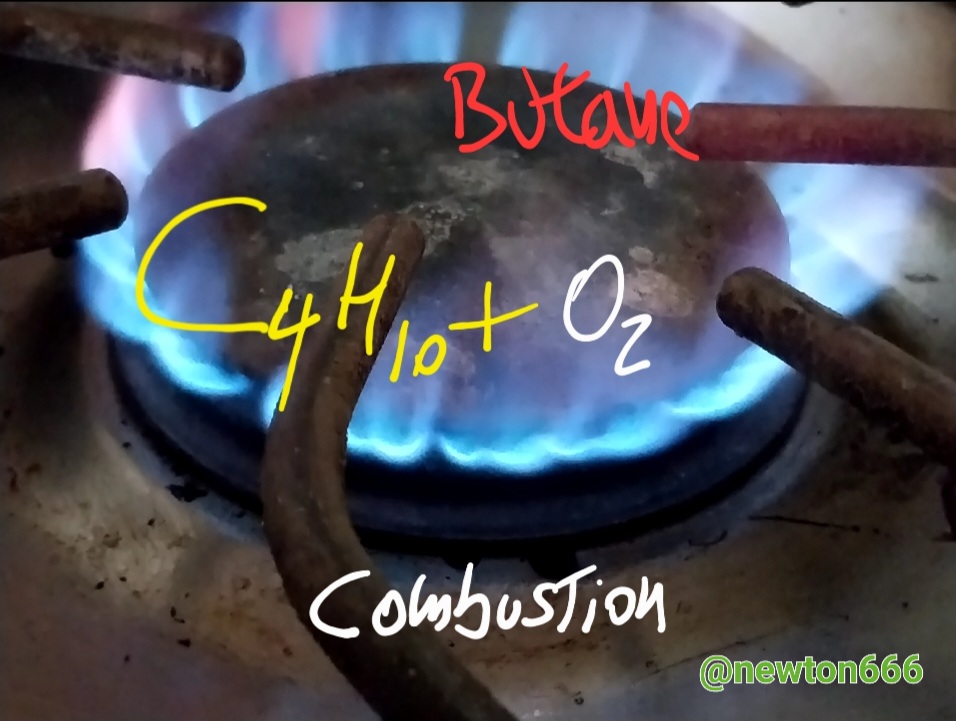
Thanks to all this information I now know why the flame is blue in the kitchen, that is thanks to the combustion of the gas with the combination of oxygen, in the case of butane this gas also makes this physical and chemical phenomenon possible also through combined with oxygen, butane is C₄H₁₀, the singular thing about this in my case was the following, mercaptan sulfide is added to this gas as an odorant compound, now I understand why this very particular characteristic apart from physical properties is also appearance Colorless, where its density is 2.52 kg/m³; 0.00252 g/cm³, Molar mass 58.08 g/mol, melting point 134.9 K (−138 °C) with a boiling point of 272.7 K (0 °C), a very interesting fact dear reader It is when butane combustion occurs that it releases a quantity of nitrogen dioxide, a toxic gas, to think that it is an energy source, it has this detail that is not favorable for our natural ecosystem. Unlike propane, butane has two options as alternatives, to obtain this one via oil distillation and the other via the fractionation of natural gas, which is why it is considered to have more energy use, since its Combustion produces more heat, thanks to all this it is good to know how science is contained in flame and in its combustion as part of physical and chemical phenomenon every day our homes are a source of valuable information to discover.
Bibliographical References
Antoine Laurent Lavoisier, the fire investigator/ Antoine Laurent ...
By Horacio Garcia, 2008.

Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Nice class about combustion!! Now how does it work the spark element , which ignites the combustion ?

!1UP
Greetings, the spark is a stimulus for combustion, since it is the initial ignition focus.
You have received a 1UP from @gwajnberg!
@ccc-curator, @stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.