New breakthrough could accelerate development of green fuel cells
Fuel cells are electrochemical energy conversion devices similar to a battery, but with the difference that they are designed to allow the continuous flow of fuel and an oxidant, which undergo a controlled chemical reaction, giving rise to the reaction products and supplying electrical energy to an external source. That is, while a battery delivers energy until the reactants are exhausted, fuel cells can continuously supply the reactants.
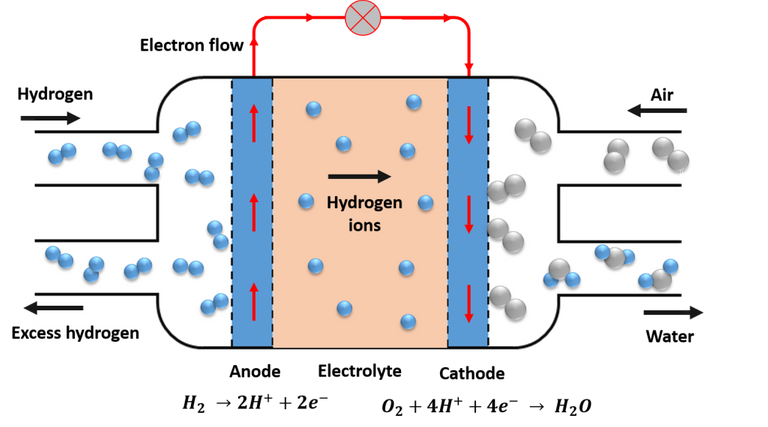
Scheme of operation of a fuel cell. Source: Image elaborated in powerpoint.
Although there are different types of fuel cells, their operation is basically the same, and the most common example is the so-called proton exchange membranes (PEM), its basic operating scheme is shown in the image above, as we can see, consist of two electrodes, In the anode the fuel undergoes oxidation and in the cathode the oxidant is reduced, these elements are separated by an electrolyte that acts as an insulator and conductor of the protons, causing the electrons to travel from the anode to the cathode through the external circuit, thus generating electricity in the process. Generally the fuel for this type of cell is hydrogen, and the oxidant is oxygen, and the advantage of this type of cell is that the only by-product is water.
In this field of fuel cells, hydroxide exchange membrane cells are emerging technologies that offer among their advantages the use of cheaper catalysts, bipolar plates and less expensive membranes than conventional proton exchange membrane (PEM) fuel cells.
This is why these fuel cells have attracted so much interest in recent years, as they can convert chemical energy directly into electricity and can be used to power zero-emission vehicles. However, one difficulty that has kept them out of the vehicle market is that their performance is severely affected by the carbon dioxide present in the air that is fed to the fuel cell to supply the oxygen needed for its operation.
CO2 rapidly decreases the efficiency of the battery by 20%, which means that the battery performs no better than a conventional gasoline engine. Practically all the CO2 that enters the fuel cells is retained by them, producing a carbonation effect with the chemical species present in the electrolyte, hindering the reactions that must take place and the conductivity of the ions.
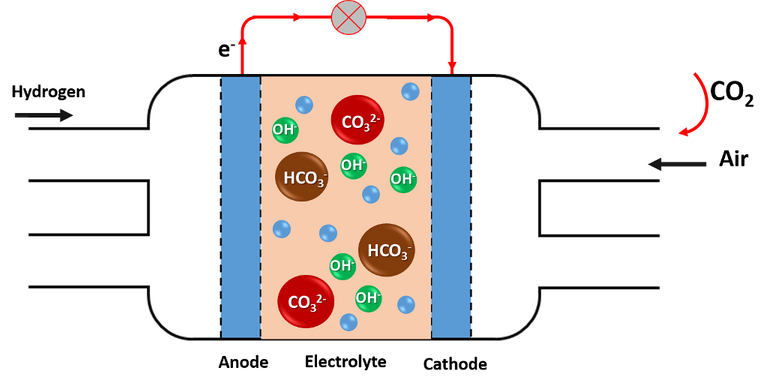
The CO2 that enters the fuel cells is retained and produces a carbonation effect on the cells. Source: image elaborated in powerpoint.
But this flaw looks like it could also provide the solution to the same problem, a group of engineers at the University of Delaware realized that fuel cells were really good at capturing carbon dioxide, and by studying the process they realized that they were very efficient at separating the incoming CO2 and preventing it from going out the other side. This team of researchers then demonstrated that they could take advantage of this separation process to place an electrochemical device upstream of the fuel cell to act as a carbon dioxide separator, capturing 99% of the carbon dioxide present in the air in a single pass. This method was recently reported in the journal Nature Energy.
This team found a way to integrate the energy source of the electrochemical technology contained in the separation membrane, and their method is to short-circuit the device internally.
By using this electrically shorted membrane, they were able to obtain a device that looks like a gas filtration membrane, but has the ability to collect carbon dioxide from the air through a more complex electrochemical process. And because it is a shorted membrane, they were able to dispense with other bulky components, which gave the researchers the freedom to build a compact spiral-shaped module, thus obtaining a large surface area in a small volume, which can make it more cost-effective and efficient for fuel cell applications.
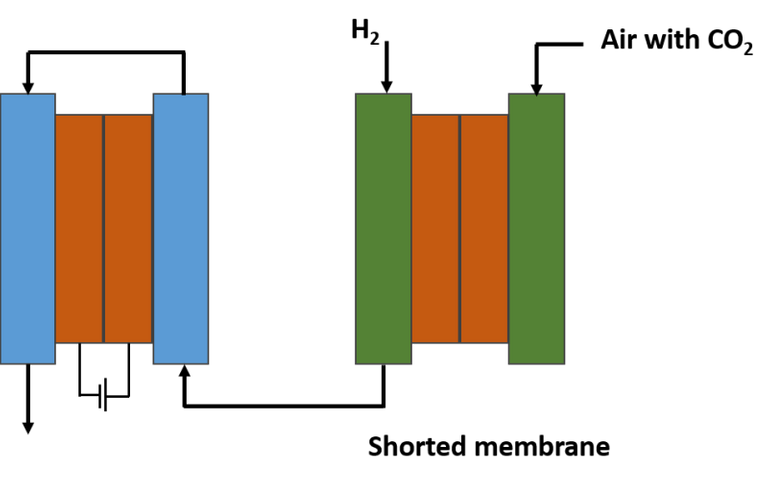
The shorted membrane would remove CO2 from the air entering the fuel cell. Source: image elaborated in powerpoint.
According to the researchers, with their initial prototype spiral cell, the size of a soda can, they were able to filter 10 liters of air per minute, removing 98% of the carbon dioxide present.
With vehicle applications in mind, the researchers estimate that the device would be about the size of a gallon of milk, and its compact, low-component design could help minimize implementation costs.
Hopefully, this device will be developed enough to make hydrogen fuel cells more efficient, a technology eager to be implemented in transportation vehicles since its only emission produced is water, and it figures to be an appropriate technology to mitigate climate change if hydrogen can be obtained in a sustainable way.
On the other hand, this development also looks promising as a carbon dioxide capture system, to be implemented in vehicles where CO2 removal and air recirculation is required, such as submarines and spacecraft, where air filtration is critical.
Thanks for coming by to read friends, I hope you liked the information. See you next time.
Electronic-terrorism, voice to skull and neuro monitoring on Hive and Steem. You can ignore this, but your going to wish you didnt soon. This is happening whether you believe it or not. https://ecency.com/fyrstikken/@fairandbalanced/i-am-the-only-motherfucker-on-the-internet-pointing-to-a-direct-source-for-voice-to-skull-electronic-terrorism
Thanks for this interesting news, which I have totally missed.
I have a question: how to make sure that we have a continuous flow of reactants? I imagine these need to come from somewhere, and this is the main limitation in this process. Am I correct?
Moving on with my thoughts in that direction, we should then be careful before claiming that those cells are totally zero-emission devices. We indeed should probably account for the extraction process of the reactants, at least for handling a fair environmental budget. Do you have (quantitative) information on this?
Now, I will also come back to the main topic of this blog, and say that the breakthrough you describe is a very cool one. It indeed addresses several issues in one shot (CO2 capture and more efficient cells) and paves te way to future better applications!
Cheers!
Hi friend @lemouth, thanks for stopping by to read, it really looks like a solution capable of addressing those two problems at the same time. And yes, in addition to the efficiency of the cells that could be solved with this separator, is that currently the main source of hydrogen are fossil fuels, so we could not yet speak of a net balance equal to zero emissions, I do not have at hand the inventory of emissions to produce hydrogen by this route, perhaps if in parallel also develop green hydrogen sources, obtained through renewable sources we could really have an alternative with zero emissions. Greetings my friend!
Thanks for coming back to me. I will try to take some time to do some research during the week-end, although this is not guaranteed as I have already quite a full agenda for both days. If you see something in the meantime, please do not hesitate to share it with me.
Cheers!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thanks for the support friends!
Your content has been voted as a part of Encouragement program. Keep up the good work!
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for new Proposal
Delegate HP and earn more
Thanks!!