Traumatic hydrofluoric acid
Okay, so just a little segway because something happened at work recently. This may be a semi rant because it took 2 accidents before management finally listened and replaced the dispenser for our hydrogen fluoride/hydrofluoric acid (HF).

This is the bottle of HF we use in the lab. It has a dispenset attached to it, but that was the thing that broke off while in use.
So for a quick background, HF is an acid we use in the laboratory. In my lab, we use this solely in four acid digestion. As to why is because HF is highly reactive to the silica in most minerals.
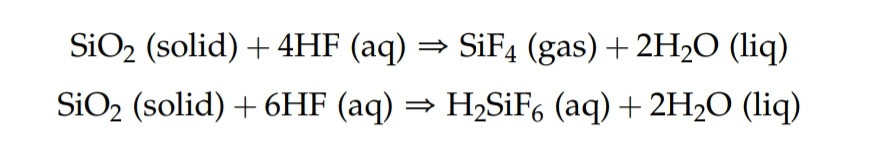
As seen from this example from the analytical chemistry book I use (Harris 8th edition), Solid silicates react with hydrofluoric acid to produce Hexafluorosilicic acid Fluorosilicic acid
It’s also unique in a that it can dissolve many metals and semimetal oxides, yet to use it alone would be ineffective like... it wouldn't completely dissolve the solids/powdered sample when used as is. That's why it’s always used in tandem with nitric acid (HNO3) and perchloric acid (HClO4).
It’s a precursor to basically all fluoride containing compounds available in the market, but it’s mostly used in the industry like in Pharmacy, geochemistry, etc. though a larger percentage is used to make toothpaste, teflon, refrigerants (freon) and your chlorofluoro carbons, and some polymers (like teflon). It’s an interesting material yes, but only if the gas and liquid form wasn’t so so so hazardous. Naturally though, it’s stable. This one exists as fluorite in nature. That’s Calcium difluoride (CaF2) and it’s actually quite pretty and stable.

But once you treat this mineral with sulfuric acid, that’s how you extract HF gas. It’s crazy how this mineral, once processed, could be deadlier than other acids.
Like if you compare it to other acids, they all look the same, yes. Clear, colourless fuming liquid with that sharp smell so it’s hard to differentiate when it’s next to other acids, the difference here is that it melts glass. It’s why it’s so often used in the glass (used to make frosted glass) and metal etching industry. And the fact that when it spills on you, you won’t feel the burn. you’d barely feel it at all at first.
Imagine, something considered a weak acid (because of its low dissociation constant) could be much much more hazardous than what’s considered a strong acid.
How it becomes hazardous is because it won’t burn, it’ll bind to your skin, your cells, your bones.
By component, you can see that HF will dissociate into a hydrogen cation (H+) and a fluoride anion (F-). And although the dissociation is slow, it will still dissociate because of how fluoride ions are so readily absorbed by your cells. It’s highly electronegative, and whatever other electronegative elements keeping your cells in condition can and will get replaced with fluorine. But more importantly, what the fluoride wants to bind to are your electrolytes. Specifically, the calcium in your bones, also to the magnesium, potassium and sodium in your blood. It has such a high affinity to calcium because that’s the element it naturally occurs with in nature. So your problem here next is that when the fluoride ion binds with the calcium in your bones, you get crystals growing inside you.
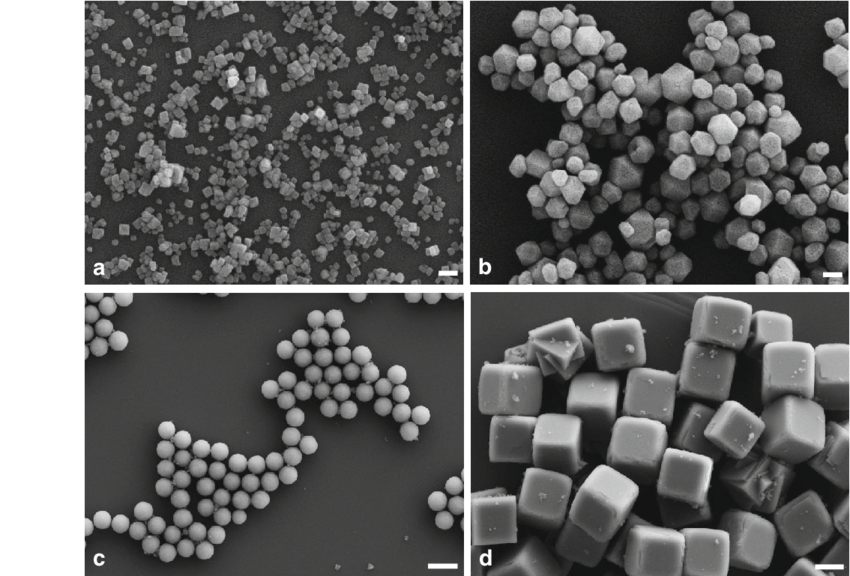
This is the CaF2 microcrystals that can be found in dental products and you can use this as a basis of what the CaF2 crystals would look like if it got in your bady, as well as the other salts the Flouride ion can bind to (side note: a reason why you shouldn't swallow toothpaste)
Source: https://www.researchgate.net/figure/SEM-images-of-calcium-fluoride-particles-prepared-by-precipitation-from-NaF-and-CaCl-2_fig2_261221836
I mean, a crystal growing in me would be cool, but if only it won’t kill me through blood clotting or through internal bleeding/lacerations.
And about the hydrogen cation part, well, that stays in the affected area but! It will still produce some swelling. Usually one would feel it a few hours after they experienced the spill.
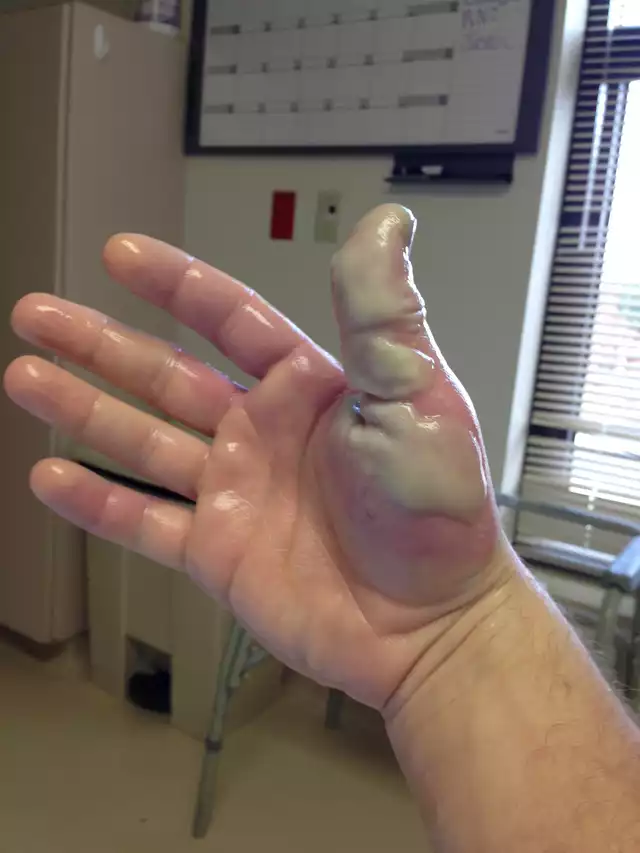
This is an example of an HF burn if left untreated. this is similar to what happened with my other workmate that only got hit on the arm. We thought it'll be fine since we had toothpaste as first aid and she washed her arm for a good 10 minutes. But what we didn't know was that her lab gown got soaked too and we only noticed her acid burn when she took off her lab gown. Her upper arm swelled and we had to rush her to the ER too. Crazy how this all happened when the week was supposed to be done.
Source: https://www.reddit.com/r/WTF/comments/20y537/my_coworker_took_a_dose_of_hydrofluoric_acid_to/
And I remember my professors telling us about a student that had an HF spill incident but did not report it. The acid used was at 40% too, so relatively pure. And it was so scary because the next day, the school received a call from the parents to inform them of the guy’s death from internal lacerations and swelling on his leg. It was hella scary so now I kind of have this deep trauma with that acid. Like from the liquid itself to the fumes because even the fumes can cause extreme lung damage, pulmonary edema. Idk it just scares me.
And another thing. Since the chemistry population in my country is small, we don’t have enough chemist doctors that know how to treat acid burns so our kinds of burns are usually dismissed. Like what happened with my 2 coworkers earlier today.
So the first aid there was to flush the affected area with water for a good 20 minutes. Luckily there was an abundance of eye showers and faucets and sinks in the digestion room so they could both flush off the acid from their skin and clothes. Luckily there was toothpaste around cuz that’s what you can use as first aid for this kind of acid spill but it's better to use calcium glucomate, but toothpaste is the more available option. That or milk with high calcium. Hella was scary to see that today because we had to rush them to the emergency room to get some blood tests done. A lethal dose is at 20 mg/kg and hopefully the results they’ll give tomorrow won’t end up being that high because I do not want to lose a coworker like this. More flushing was done in the ER but with an electrolyte solution. It was just crazy. So now, the purchasing department finally went and bought more face shields and acid gloves to handle this acid better. They also finally looked into buying a better dispenser. Honestly. They had to wait for an accident this severe before they finally looked into getting a safer dispenser.
—------
So this is my science ramble for today. It wasn’t really purely ranting cuz I do think there’s plenty of take away points to be taken from this experience of mine. But still. Today was traumatizing, I swear.
Sources:
https://pubchem.ncbi.nlm.nih.gov/compound/Hydrofluoric-acid#section=InChI
https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750030.html
https://doi.org/10.1016/B978-0-444-59550-8.00002-8
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4098557/
my own experience with handling this shit
Posted with STEMGeeks
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Yay! 🤗
Your content has been boosted with Ecency Points, by @bananzell.
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for new Proposal
Delegate HP and earn more
Congratulations @bananzell! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 25000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts:
Support the HiveBuzz project. Vote for our proposal!
Oh dang. That burn looks nasty.
!discovery 37
That one I saw on Reddit is. The one my coworker got peeled already and it was so gross. The flesh basically got melted
This post was shared and voted inside the discord by the curators team of discovery-it
Join our community! hive-193212
Discovery-it is also a Witness, vote for us here
Delegate to us for passive income. Check our 80% fee-back Program
this is really informative ty.
do these crystals formed inside the body decay?
The crystals don't really decay. They don't really dissolve either. It's pretty stable and that's why it'll either cause clotting or it'll keep causing lacerations on your blood vessels
😖