Inverse Functions - Inverse of Radioactive Decay Example
Last video I did an example on radioactive decay when given the half life of strontium-90 and derived an exponential function. In this video I go over what the inverse of that function is as well as how to interpret it.
Watch video on:
- BitChute:
- 3Speak:
- DTube: https://d.tube/#!/v/mes/zs3yzqk5ar8
- YouTube: https://youtu.be/jQhzBidLARo
Download video notes: https://1drv.ms/b/s!As32ynv0LoaIiOADbr1KLXzSh8rxig?e=b9pOtP
View Video Notes Below!
Download these notes: Link is in video description.
View these notes as an article: https://peakd.com/@mes
Subscribe via email: http://mes.fm/subscribe
Donate! :) https://mes.fm/donate
Buy MES merchandise! https://mes.fm/storeReuse of my videos:
- Feel free to make use of / re-upload / monetize my videos as long as you provide a link to the original video.
Fight back against censorship:
- Bookmark sites/channels/accounts and check periodically
- Remember to always archive website pages in case they get deleted/changed.
Buy "Where Did The Towers Go?" by Dr. Judy Wood: https://mes.fm/judywoodbook
Subscribe to MES Truth: https://mes.fm/truthJoin my forums!
- Hive community: https://peakd.com/c/hive-128780
- Reddit: https://reddit.com/r/AMAZINGMathStuff
- Discord: https://mes.fm/chatroom
Follow along my epic video series:
- #MESScience: https://mes.fm/science-playlist
- #MESExperiments: https://peakd.com/mesexperiments/@mes/list
- #AntiGravity: https://peakd.com/antigravity/@mes/series
-- See Part 6 for my Self Appointed PhD and #MESDuality breakthrough concept!- #FreeEnergy: https://mes.fm/freeenergy-playlist
- #PG (YouTube-deleted series): https://peakd.com/pg/@mes/videos
NOTE #1: If you don't have time to watch this whole video:
- Skip to the end for Summary and Conclusions (if available)
- Play this video at a faster speed.
-- TOP SECRET LIFE HACK: Your brain gets used to faster speed!
-- Browser extension recommendation: https://mes.fm/videospeed-extension
-- See my tutorial to learn more: https://peakd.com/video/@mes/play-videos-at-faster-or-slower-speeds-on-any-website- Download and read video notes.
- Read notes on the Hive blockchain #Hive
- Watch the video in parts.
-- Timestamps of all parts are in the description.Browser extension recommendations:
- Increase video audio: https://mes.fm/volume-extension
- Text to speech: https://mes.fm/speech-extension
Inverse Functions: Example on Inverse of Radioactive Decay Equation
Example
From my last video: http://youtu.be/dmMRX82zqlc
The half-life of strontium-90, 90Sr, is 25 years. This means that half of any given quantity of 90Sr will disintegrate or decay in 25 years.
(a) If a sample of 90Sr has a mass of 24 mg, find an expression for the mass m(t) that remains after t years.
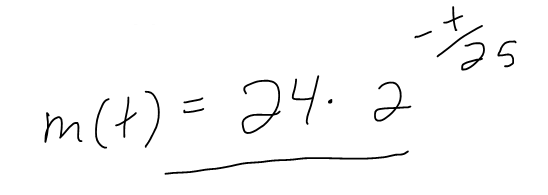
(b) Find the inverse of the above equation and interpret what it means.
Solution
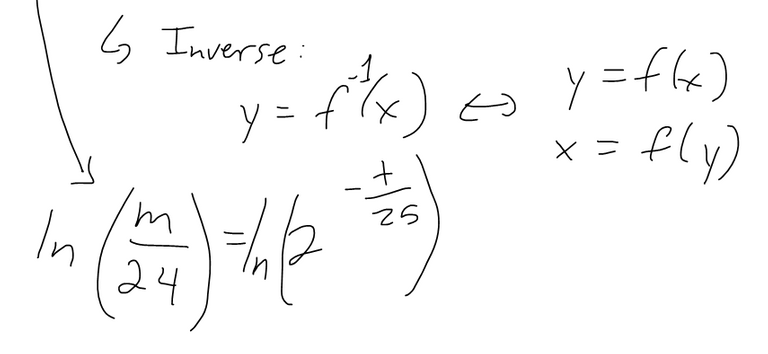
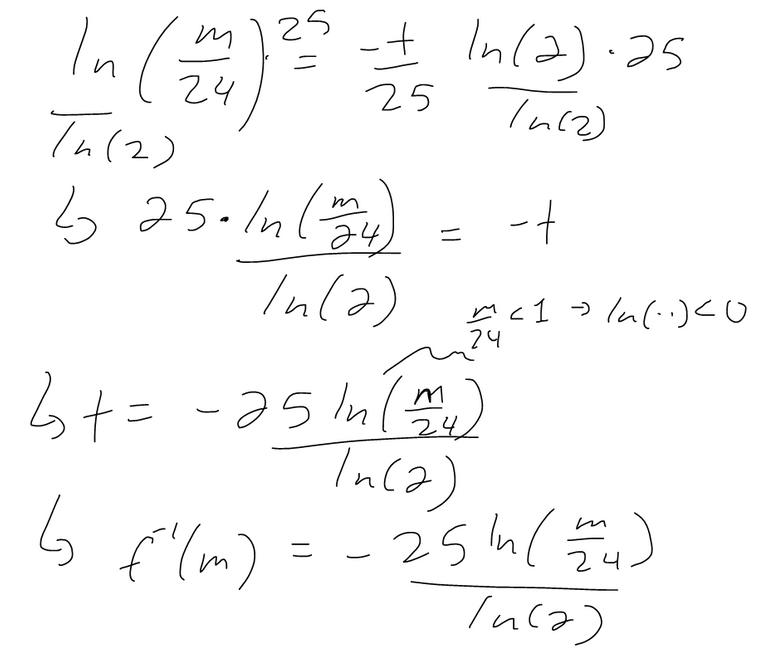
What this equation means is that it is the time required for the strontium-90 to decay to m milligrams.
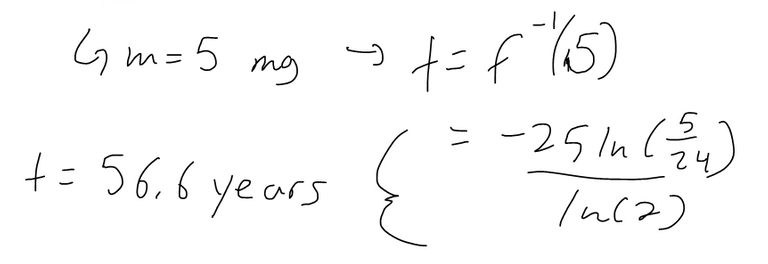
hello @mes,
all the formulas took me back to university, when I was studying chemistry I didn't think I would find people who like formulas, thanks for the good information.
publicity note:
I'm promoting That curation trail on HIVE and it would help me a lot if you forward it, You can also check it yourself: you might find it interesting to join, I'd love to hear your comments.
Yup, formulas was always second nature to me!
And thanks for the info, I haven't been on a Curation Trail but will check it out!