Colligative properties of solutions. Study of the increase in boiling point
Hello Hive scientific community!
All these properties are characterised by the fact that they depend only on the relative amount of solute and solvent but not on their chemical nature and are known as colligative properties.
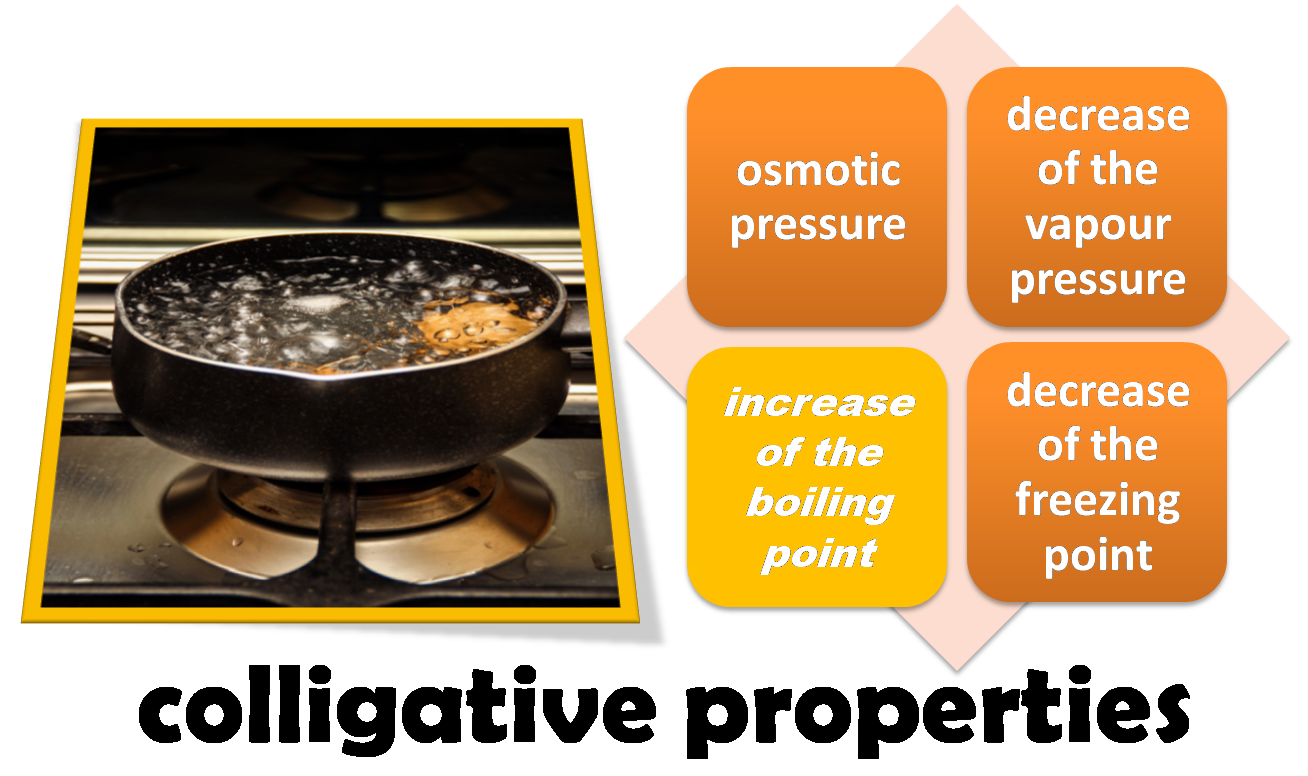
Source:Image made in power point with image of public domain1
For the study of colligative properties it is very important to master the units of concentration with which we work, such as mole fraction and molality. These units are given by:
- Mole fraction (X): expresses the ratio of the number of moles of a substance to the total number of moles of all the species present in the mixture. It can be calculated by the following expression:
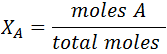
- Molality (m): expresses the amount of solute in moles present in one kilogram solvent, and can be calculated by the following expression:
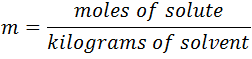
We will begin with a study of the fundamentals of one of these properties, namely boiling point elevation. I invite you to read!
Fundamentals of boiling point increase
Solvent boiling point
It is important to define the boiling point as the temperature at which the vapour pressure of a liquid is exactly equal to the applied vapour pressure. If we refer to normal boiling point, it is the temperature at which the vapour pressure of a liquid is exactly equal to the pressure of 1 atm (760 torr). The vapour pressure of water is 760 torr at 100°C, its normal boiling point[3].

Boiling point of water. Source: wikipedia.org
You may have heard that the higher you are, the lower the pressure. This means that if we are for example on a mountain above sea level and we boil water in a pot, we can expect that it will boil at less than 100 °C and that we will use more charcoal to cook the food. It is also very common to use pressure cookers in our kitchens as they cook faster because the water boils at a higher temperature at higher pressures.
Now, when a liquid is heated its vapour pressure increases, and if the temperature is high enough steam bubbles begin to form below the surface, which rise and burst, releasing steam into the air. If the vapour pressure of the bubbles is lower than that applied to the surface of the liquid, they burst and boiling does not occur.
Boiling point of the pure solvent when adding a non-volatile solute
When a non-volatile solute is added to a pure solvent, its vapour pressure decreases, so the solution must be heated to a temperature higher than that of the solvent so that its vapour pressure is equal to atmospheric pressure.This is the basis for the colligative property of boiling point increase.
Mathematically it can be expressed according to Raoult's Law, which states that the rise in the boiling point of a pure solvent caused by the presence of a non-volatile solute is proportional to the number of moles of solute dissolved in a given weight of solvent.
It is therefore expressed as:
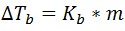
Here, the term ∆Tb refers to the increase of the boiling point of the solvent, which is given by the difference of the boiling point of the solution minus that of the pure solvent, m is the molality of the solution and Ke is a constant called the molal constant of boiling point elevation and which varies according to the solvent, it does not depend on the type of solute.
Here are the values of some of the constants for different solvents
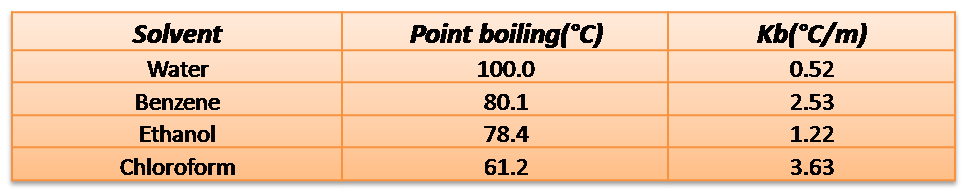
Source: Data taken from Atkins (2006)
Experimental application of boiling point increase
In chemistry, one of the defining properties of a substance is its molar mass, which is given by the mass per mole of its molecules and is expressed in g per mole (g/mol). To theoretically calculate the molar mass of a compound we must know the chemical formula of the substance, and it is given by the sum of the molar masses of the elements that form the molecule[1], so for example for H2O the molar mass is 18 g/mol.
At laboratory level, the following experimental procedure based on boiling point elevation can be used for molar mass determination, in this case using urea as a non-volatile solute.
- Materials and equipment:
Beaker, graduated cylinder, thermometer, volumetric balloon, glass stirrer, boiling beads, balance and heating plate.
- Experimental procedure:
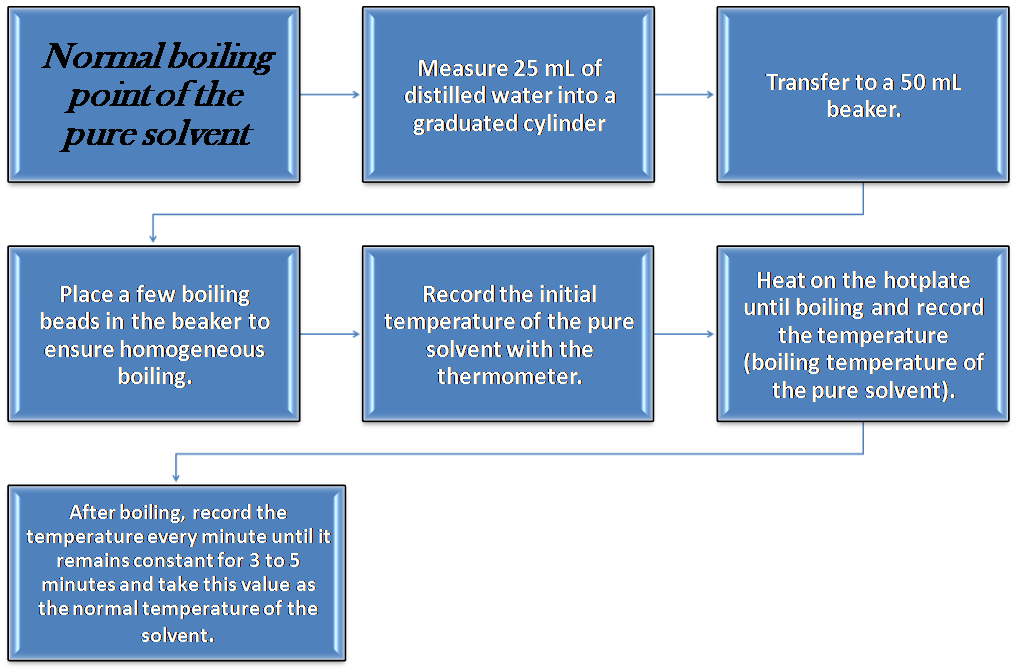
Part I: Determination of the normal boiling point of the pure solvent
The steps consist of:
Source: @yusvelasquez
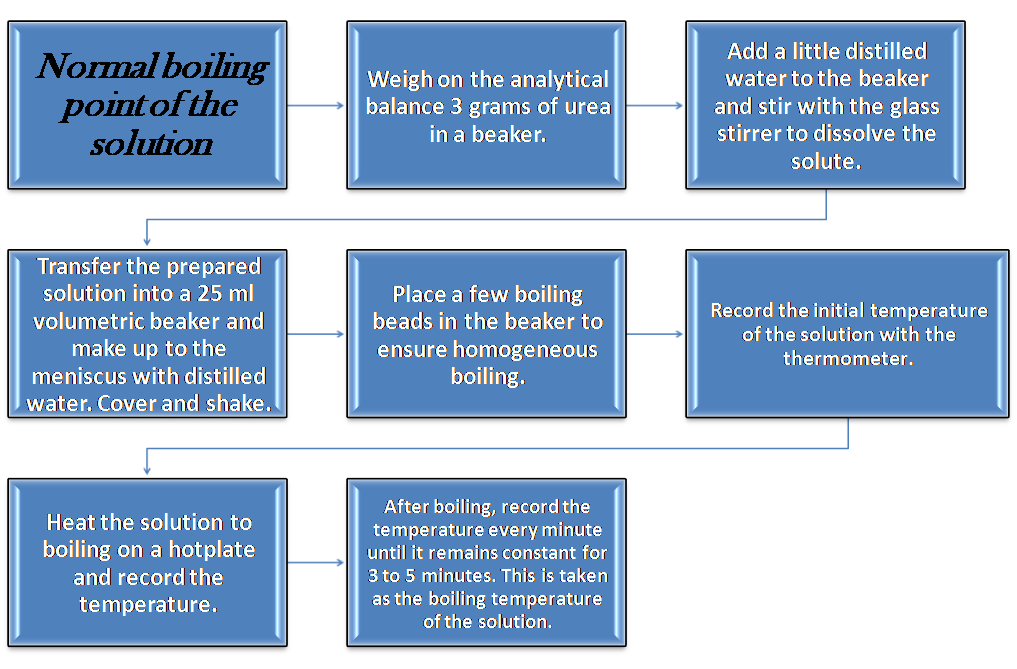
Part II: Determination of the normal boiling point of the solution
The steps consist of:

Source: @yusvelasquez
Calculations
For the determination of the molar mass of urea the equations used are:
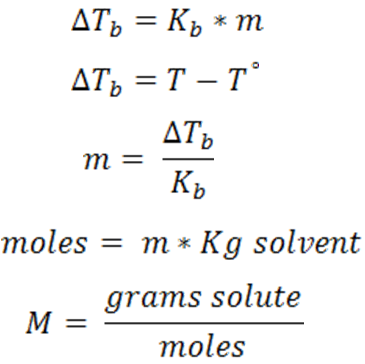
Where:
T = boiling temperature of the solution
T° = boiling temperature of the pure solvent
M = molar mass
m = molality
Mathematically the colligative properties can be simplified and facilitated by using the unit of molality concentration.
As we can see, the study of colligative properties, in this specific case the elevation of the boiling point, is important at a practical level, the experimental procedure is simple based on the measurement of temperatures, so it is necessary to work with great precision. The calculation of the molar mass of a compound is an important step in the characterisation and identification of substances.
I hope you find the presented information useful, see you next time!
References
- Atkins, P. and Jones, L. (2006). Principles of Chemistry. Pathways to discovery. Panamericana
- Whitten K and Gailey K. (1985). General Chemistry.) Interamericana
- Brown, T. and Lemay, H. Chemistry. The central science. Prentice Hall
- Practical Manual. UNEFM
Nice class and introduction of concepts like Molarity (I guess you wrote Molality in the end, but it was just a lack of attention I guess!) But well done!!!
!1UP
Hi friend, thanks for stopping by and reading and paying attention to the equations, but in this case the equations are based on molality as a unit of concentration.
You have received a 1UP from @gwajnberg!
@stem-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.
PIZZA Holders sent $PIZZA tips in this post's comments:
@curation-cartel(10/20) tipped @yusvelasquez (x1)
Learn more at https://hive.pizza.