Application of precipitation volumetry in the determination of chlorides in a food sample.

The determination of chloride ions, expressed as sodium chloride, is one of the fundamental methods used for foodstuffs due to the implication of salt in consumption and its influence on the organoleptic characteristics of the product, its importance lies in the fact that table salt (sodium chloride) is one of the most widely used additives in the food industry.
As we know, salt has the function of enhancing flavors, so its control is decisive to maintain the organoleptic characteristics of the sauce, especially the flavor, so it is very common to associate the overall taste of food with its salt content, and the product can be tasteless if its content is too low or very unpleasant if it is exceeded.
Another importance of controlling the salt content is related to the function of sodium chloride in food preservation, favoring its preservation; we already know that salting meats is a practice widely used for their preservation without refrigeration.
The chloride ions present in tomato sauce are derived from the incorporation of salt during its preparation. For its preparation, previously selected, washed and peeled tomatoes are crushed and sautéed in vegetable oil together with other ingredients, then the resulting paste is transferred to a tank where, with constant agitation, salt and sugar are sprinkled and the agitation continues for some time. The amount of tomato sauce to be produced is taken into account to add the kilograms of salt and sugar[1].

Figure 2. Commercial tomato sauce. Source: @yusvelasquez
Fundamentals of Mohr's method
The method used to determine the concentration of the analyte is known as Mohr's method, in which the chloride ions present in the sample are precipitated by reacting them with the silver ions present in a standard solution of silver nitrate after neutralization with sodium hydroxide, during a volumetric titration, using potassium chromate as an indicator. The end point of the titration is determined by the appearance of a red precipitate of silver chromate. The reactions involved in the titration are:
Titration reaction:
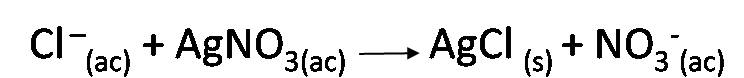
Indicator reaction:
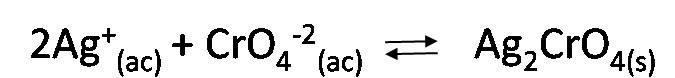
To detect the final pint of the titration it is necessary that precipitation of the indicator occurs near the equivalence point. When silver ions are added to a solution containing a high concentration of chloride ions and low concentration of chromate ions, the silver chloride will precipitate first; the silver chromate precipitate will not form until the silver ion concentration increases sufficiently to exceed the Kps of Ag2CrO4[3].
To carry out this titration it is necessary to control the pH of the solution in a range between 7 and 10. This is because if the pH is very acidic, the chromate concentration decreases and for the appearance of the precipitate to be possible at the equivalence point it will be necessary to add an excess of silver nitrate, leading to errors in the titration.
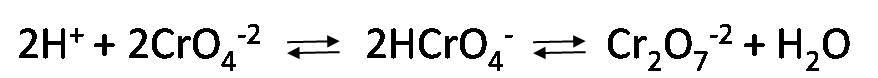
On the contrary, if the pH is very basic, it will favor the appearance of silver oxide precipitates of black color masking the end point.
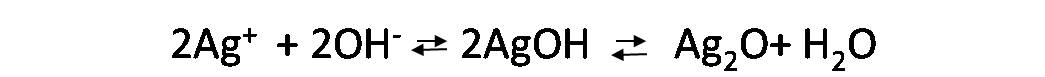
Experimental procedure
The determination of chlorides in the samples is carried out by taking aliquots of the diluted product following a procedure adapted from COVENIN 1193-81[4].
Materials and reagents
- 200 mL Erlenmeyer flask
- Graduated cylinder of 100 mL
- 100 mL beakers
- 2 mL pipettes
- 25 mL burette
- Potassium chromate solution, K2CrO4, 0.01 M
- Silver nitrate standard solution, 0.1 M
- Sodium hydroxide 0.1 M
The following steps should be followed to apply the method:
Sample preparation
- Weigh 5 g of tomato sauce sample in a beaker, add 50 mL of distilled water, homogenize by stirring and let it stand for one hour.

Figure 3. Tomato sauce sample. Source: @yusvelasquez
Titration of the sample
- Three aliquots of 5 mL of the sample are taken.
- By means of the addition of the sodium hydroxide solution the pH is adjusted between 7 and 10.
- 1 mL of potassium chromate is added to each sample.
- It is proceeded to value with the standardized silver nitrate solution, until the appearance of a reddish-brown precipitate.

Figure 4. End point of titration, appearance of brick red silver chromate precipitate.
- Record the volume of silver nitrate spent in the titration.
- A blank assay is also performed: to 25 mL of distilled water placed in a 250 mL beaker, the same procedure described from step 2 is applied.
Calculations
The following table shows the data obtained experimentally in the titration of a 5.034 g sample of a commercially available ketchup sauce.

According to COVENIN 75:1995[5], the chloride content in tomato sauce is expressed as % w/w NaCl, and is calculated as follows.
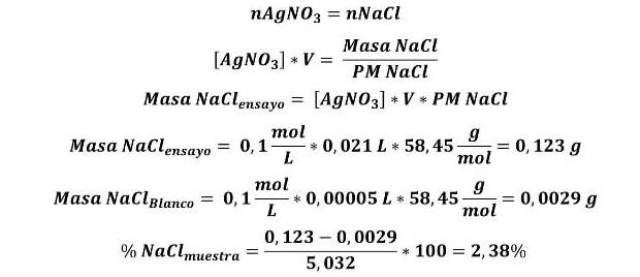
According to the results, 2.38 % NaCl is obtained in the sample. The COVENIN 75:1995 standard establishes that the maximum chloride content is 4% w/w of NaCl, so it can be said that the sample of the sauce evaluated complies with the aforementioned standard.
Conclusion and contribution
As we have seen, the determination of chlorides by Mohr's method turns out to be quite accurate, it allows us to quantify and control the amount of salt added to food, since, as we have already mentioned, it is not only about improving the taste of the product but this parameter also influences other quality characteristics, in addition to the need to comply with the normatively exposed in order to ensure the health of consumers.
The knowledge of this method and its principle is very important for the student and the professional of chemical analysis, since it is a fundamental method in the quality control of many foods and a widely used method in quality control laboratories.
And according to the example used, we can say that the concentration of the analyte is within the parameters established in the respective food standard that refers to the content of chlorides in tomato sauces.
Well friends, this is the end of this post, I hope you find the information presented here useful. Remember the importance of analytical methods in food quality control, see you next time!
References
- Martínez, I., Blanco, R. Determination and comparison of physicochemical parameters of tomato sauces obtained in supply centers of the city of León. National Autonomous University of Nicaragua-León. Online document
- Alimentacionsana.com. Ketchup sauce.
- Ramírez, D., Velásquez, Y., Lugo, O., Ferreira, M. Practical guides of the Laboratory of Analytical Chemistry UNEFM.
- COVENIN 1193-81. Foodstuffs. Chloride determination.
- COVENIN 75:1995. Tomato sauce.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.
Excelente explicación, El método Mohr es muy preciso, esto nos indica que nuestro cuerpo recibe lo necesario en cloruro de sodio.
Gracias por esto @yusvelasquez
Hola @nathyortiz!
Los métodos analíticos son muy precisos en la cuantificación de distintos analitos. Gracias por leer y comentar!