Understanding the Dynamic Roles of Metalloenzymes and Metal-Activated Enzymes |ChemFam #70|
Greetings to everyone! Enzymes are like the conductors of the intricate orchestra of life, orchestrating biochemical reactions that keep living organisms humming along. Among these conductors, there are two special kinds: metalloenzymes and metal-activated enzymes. They're like the rockstars of the enzyme world, relying on metal ions to add that extra flair to their performances and contribute to the magic of biological processes.
The natural catalysis in biological systems are enzymes. The enzymes are basically macromolecules with the peptide chains. About 30% of the enzymes are metalloenzymes or metal-activated enzymes. These are commonly called metalloenzymes without making any distinctions. The biocatalysts have the main frame work built of proteins, but in metalloenzymes the activity depends on the presence of the desired metal ion. Thus the metalloenzymes have two structural components: the protein portion described as apoenzymes and a small non-protein prosthetic group which may be a simple metal ion or a complexed metal ion. Such enzymes having both protein part and non-protein part are called holoenzymes.
The apoenzymes and the prosthetic group work jointly, though the prosthetic group resides at the active site. The selectivity and the catalytic efficiency largely depend on the protein structure. In fact presence of the metal ion is not the sufficient condition for enzymatic activity. Thus catalysis is an reciprocal effect. The role of protein in enzymatic activity can be illustrated by considering the metal ion catalysed hydrolysis of ATP. In vitro, the hydrolysis of ATP does not change appreciably from metal to metal, but in the enzymatic hydrolysis of ATP, Mg(II) and Mn(II) show the catalytic effect but Cd(II), Ni(II) and Co(II) show the inhibitory effect. The metal ion catalysis arises when the metal ion preferably binds with the phosphate group. Ni(II), Co(II) and Cd(II) probably block some sites which are needed for catalysis while Mg(II) or Mn(II) does not block sites. It indicates that the protein chain largely regulates the enzymatic activity of metalloenzymes.
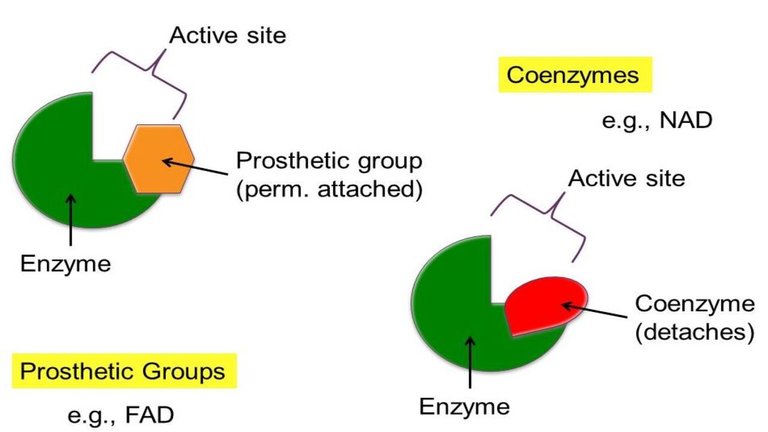
In the enzyme catalysis, the other two terms cofactor and coenzymes are also important. Sometimes, a particular component of low molecular weight gets reversibly bound to an enzyme to carry out a particular reaction and after the reaction, it gets detached from the enzyme and combines with another enzyme to activate it. Such a group is describes as coenzyme (e.g. B12 coenzyme). The prosthetic group and coenzymes are described by the general term cofactor. Thus in metalloenzymes, cofactor contains the desired metal ion. In general if an enzyme requires a non-protein entity for its activity, then it is referred to as a holoenzyme which consists of an apoenzyme and a non protein part.
Metalloenzymes: A Symphony of Metal Ions and Biomolecules:
Metalloenzymes are enzymes that require metal ions for their structural stability and catalytic function. The metal ions within these enzymes can serve as cofactors, essential for their proper folding and activity. The classification of metalloenzymes is based on the type of metal ion present, and common examples include zinc, iron, copper, and manganese.
Some representative examples of metalloenzymes of different classes are given below-
(a) Metallohydrolases: Carboxypeptidase (Zn2+, hydrolysis of C terminal peptide residues), Urease (Ni2+; hydrolysis of urea), α-amylase (Ca2+, Zn2+; hydrolysis of glucosides).
(b) Metallooxidoreductases: Oxygenase (Fe; reactions of dioxygen O2 with the organic substrates in which oxygen atoms are incorporated into the final oxidized products), cytochrome c oxidase (Fe, Cu), catalase and peroxidase (Fe), nitrogenase (Fe, Mo, V; nitrogen fixation).
(c) Metalloisomerases and metallosynthases: Co-containing vitamin B12 coenzyme.
(d) Metallolyases: Carbonic anhydrase (Zn2+, hydration of CO2 and dehydration of H2CO3.
Metal-Activated Enzymes: Dynamic Modulation of Catalytic Activity:
Metal-activated enzymes, in contrast to metalloenzymes, do not necessarily require metal ions for their structural integrity but exhibit enhanced catalytic activity in the presence of specific metal cofactors. These enzymes undergo conformational changes upon metal binding, leading to increased substrate affinity and catalytic efficiency.
One prominent example of a metal-activated enzyme is carbonic anhydrase, which accelerates the interconversion of carbon dioxide and bicarbonate. The enzyme's catalytic efficiency is significantly enhanced upon binding to zinc ions. The dynamic regulation of metal-activated enzymes allows cells to fine-tune their metabolic pathways in response to changing environmental conditions.
Biomedical Implications:
(A) Metalloenzymes in Health and Disease:
Metalloenzymes play crucial roles in maintaining cellular homeostasis, and disruptions in their function are associated with various diseases. For example, alterations in the activity of metalloenzymes like superoxide dismutase have been linked to neurodegenerative disorders such as amyotrophic lateral sclerosis (ALS).
(B) Therapeutic Targeting:
Understanding the intricate details of metalloenzymes and metal-activated enzymes opens avenues for therapeutic interventions. Metal-based drugs that target specific metalloenzymes are being explored for the treatment of diseases, such as the use of platinum-based compounds in cancer chemotherapy.
Role of metal ions in metal-protein systems and metalloenzymes:
Metal ions play crucial roles in metal-protein systems and metalloenzymes, influencing their structure, stability, and catalytic functions. These interactions are fundamental to various biological processes, and understanding the roles of metal ions in these systems is key to unraveling the mysteries of cellular function.
The non-transition metal ions like Na+, K+, Ca2+ and Mg2+ are responsible for a large number of biological activities like nerve impulse transmission, muscle contraction, secretion of hormones etc. These activities mainly depend on the selective distribution of the cations across the membrane. A breakdown of the membrane's selectivity can act as a trigger to initiate the different types of biochemical events.
Some metal proteins can participate in storage and transport of metal ions. The metal ions can act in different ways: (a) structural context; (b) template synthesis of biomolecules: (c) blocking of some functional groups through metal coordination; (d) Lewis acid catalysis in different biological functions like hydrolytic cleavage, protein synthesis, decarboxylation etc.
Conclusive thoughts
In the grand concert of life, metalloenzymes and metal-activated enzymes are the standout performers, infusing the symphony of biochemical reactions with their metallic magic. As we dive deeper into their backstage secrets, we not only unravel the mysteries of fundamental biological processes but also discover new ways to compose innovative therapeutic melodies. So, let the dance between enzymes and metals continue, bringing harmony to the extraordinary journey of life.
Until we meet again :)

Bioinorganic Chemistry, AK Das
Cracking the Thermal Code: Differential Thermal Analysis in Modern Research |ChemFam #69|
Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
The Silent Revolution: How Polymers are Shaping Our World? |ChemFam #67|
Beyond the Bin: The Many Faces of Plastic Management |ChemFam #66|
Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
Unveiling The Control Of Chemistry: How Hormones Dictate Our Mood |ChemFam #57|
Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
PS The thumbnail image is being created by me using canva.com



This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited..
This post received an extra 1.00% vote for delegating HP / holding IUC tokens.
Thank you @indiaunited and @bhattg bhai !
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thank you @stemsocial !
Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 14000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts: