Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Greetings to everyone! Countless chemical reactions are occurring in our day to day life, from boiling an egg for your breakfast to taking up your medicines. But why do the chemical reactions takes place? Why do they react with each other? Well, the absolute answer lies in two specific terms called Thermodynamics and Kinetics. These two terms are arguably most important concepts in chemistry. Often people have various perspective to these two terms, and sometimes filled with misconceptions. Today, we shall be understanding both these terms and their implications in chemical reactivity.

Thermodynamics: The energetic stability of molecule
Thermodynamics is the branch of physics that deals with heat, work and temperature, their relation to energy radiation and physical properties of matter. Thermodynamics describes the properties of a system at equilibrium. The more stable, a compound the greater is its concentration at equilibrium. Thus, if the products are more stable (have a lower free energy) than the reactants, there will be a higher concentration of products than reactants at equilibrium, and Keq will be greater than 1. On the other hand, if the reactants are more stable than the products, there will be a higher concentration of reactants than products at equilibrium.
Suppose for a reaction,
aA + bB → cC + dD
Keq = [products] / [reactants] = [C]c [D]d / [A]a [B]b
Thermodynamics is only concerned with the final state of a system and the mechanism of transformation does not matter at all.
The thermodynamic quantity is the energy difference resulting from the free energy (ΔG) given off during a chemical reaction i.e., the stability of the products relative to the reactants. All substances tend to be in the lowest energy state. So when a substance is at lower energy, it is more stable. Thermodynamic stability has to do with the relative energies of reactants and products and it is independent of the pathway.
Kinetics: The barriers of chemical reactivity
Kinetics is the study of the experimental measurement of macroscopic properties of a reaction mixture and how the concentration of product and reactants changes over time, i.e., reaction rate. Kinetics describes the rates of reactions and how fast equilibrium is reached, it gives no information about conditions once the reaction reaches equilibrium. The quantity related to kinetics is the rate constant (k). It is associated with the activation energy required for the reaction to move forward, that is, the reactivity of the reactants.
How fast a reaction occurs is independent of the type i.e., whether a given reaction is exergonic or endergonic. Kinetic stability has to do with the pathway between reactants and products. Kinetic stability is largely dependent on the activation energy for the reaction. The activation energy is determined by the chemical potential energy of the reaction intermediate(s). If the activation energy is high then at low temperatures the reaction will be slow. It can be made faster by increasing the temperature. If the activation energy is low then the reaction will be fast even at low temperatures. Such reactions do increase their rates with increasing temperature as well.

Thermodynamics versus Kinetics
When a reaction produces more than one product, the product that is formed most rapidly is called the kinetic product, and the most stable product is called the thermodynamic product. Reactions that produce kinetic product as the major product are said to be kinetically controlled. Whereas reactions that produce the thermodynamic product as the major product are said to be thermodynamically controlled.
For most reactions kinetic and thermodynamic product are same. However to understand and distinguish both the two types of products, let us study by taking an example. The electrophilic addition to 1,3-butadiene is an example where kinetic product and thermodynamic product are not same. In this case the product that predominates depends on the conditions under wjhich the reaction is carried out.
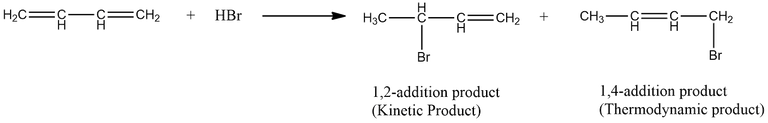
If the reaction is carried out under sufficiently mild i.e., low temperature conditions to cause the reaction to be irreversible, the major product will be kinetic product. The addition of HBr to 1,3-butadiene is carried out at -80°C, the major product is the 1,2-addition product.

On the other hand, if the reaction is carried out under sufficiently vigorous i.e., high temperature conditions to cause the reaction to be reversible, the major product will be the thermodynamic product. When the reaction is carried out at 45°C , the major product is the 1,4-addition product.

Why is the anomaly occurs?
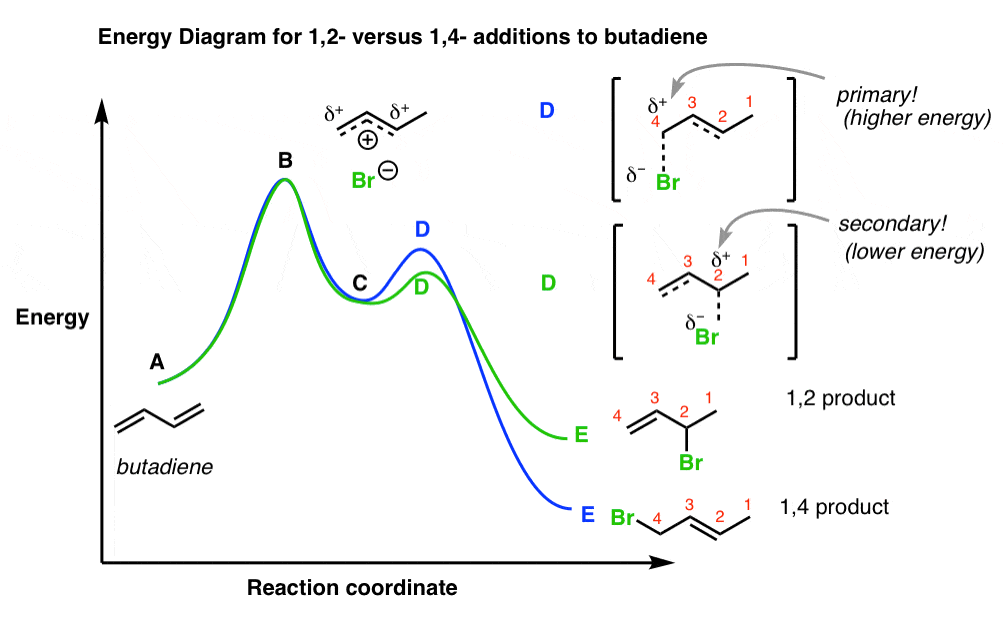
A reaction coordinate diagram helps to explain the anomaly of why different products predominate under different reaction conditions. The first step of the reaction i.e., addition of a proton to C-1 is the same whether the 1,2-addition product or the 1,4-addition product is being formed. It is the second step of the reaction that determines whether the nucleophile (Br-) attacks C-2 or C-4. Because the 1,2-addition product is formed more rapidly, we know that the transition state for its formation is more stable than the transition state of formation of the 1,4-addition product.
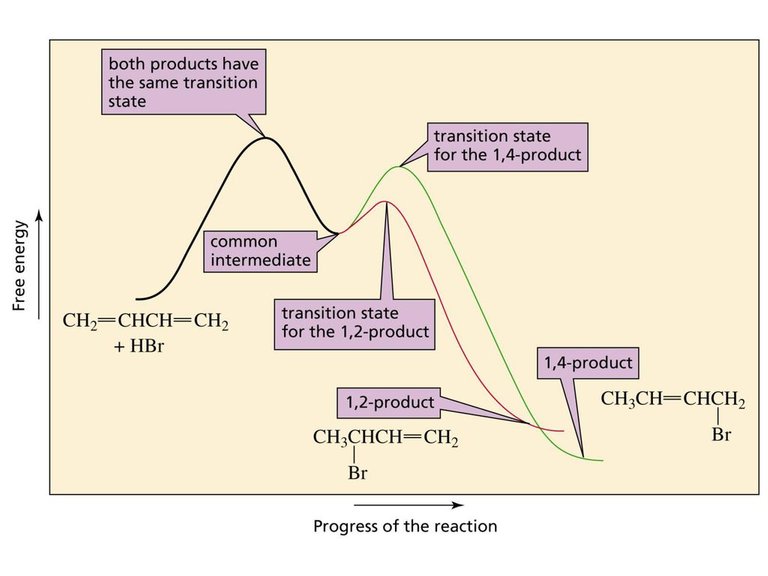
At low temperature (-80°C), there is enough energy for the reactants to overcome the energy barrier for the first step of the reaction and therefore form the intermediate, and there is enough energy for the intermediate to form the two addition products. However there is not enough energy for the reverse reaction to occur. The thermodynamic product reverses less readily because it has a higher energy barrier to the common intermediate, so it gradually comes to predominate in the product mixture.
The transition state (TS) for the 1,4-addition product has positive (+ve) charge on less substituted carbon, therefore its activation energy is large and forms slowly, but it is more stable than the kinetic product due to the formation of dominant disubstituted alkene product. In this case, the intermediate formed has a positive charge on a primary allylic carbocation.
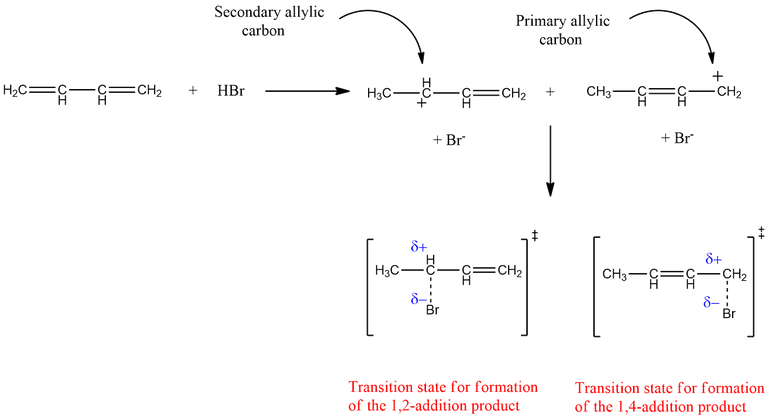
On the other hand, the transition state for 1,2-addition product has a positive (+ve) charge on more substituted carbon and has lesser activation energy and forms faster. It is less stable than the thermodynamic product due to the formation of less dominant monosubstituted alkene. In this case, the intermediate formed has a positive charge on secondary allylic carbocation.

Summarising Thermodynamics and KInetics
Thermodynamics is all about “if”
• if a process or a reaction can occur or not.
• if a systems is in stable or metastable equilibrium.
• if sufficient driving force is present to enforce a favourable transformation.
Kinetics is all about “how”
• how fast or slow a process can occur, i.e., determining the rate
• how transition from nonequilibrium to equilibrium systems, or between two equilibrium states occurs
• how to overcome the energy barrier to finish the transformation from the starting (reactant) state to the final (product) state.
Conclusive thoughts
We understood that thermodynamics determines in what direction a chemical reaction proceeds, and kinetics determines the speed or rate at which that process occurs. In all and all the term thermodynamics is related to stability and kinetics is related to reactivity. In general, at high temperature, a reaction is under thermodynamic control (reversible conditions) and the major product is the more stable system. Whereas at low temperature, a reaction is under kinetic control (irreversible conditions) and the major product is that from fastest reaction. For a reaction irreversible under mild conditions and reversible under vigorous conditions at a certain temperature one product changes to another. At this particular temperature there is change between a kinetic and a thermodynamic product.
We shall meet again :)
Smith, M. B. March's Advanced Organic Chemistry, 7th ed., p 272
Surface catalysed hydrochlorination of alkene
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
SCRAP Giveaway | Terracore | Draw #10 |
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
SCRAP Giveaway | Terracore | Draw #5 |
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
Introduction to Drugs and Drug-Target Interaction |ChemFam #42|
Scientists Analyze a Single Atom With X-Rays For The First Time |ChemFam #41|
Can We Slow Down Aging? |ChemFam #40|
Studying The Cluster Compounds: The LNCC |ChemFam #39|
PS The thumbnail image is being created by me using canva.com by using template image from J.M. Book


Although i am not an expert in chemistry i must commend the effort you put in educating us on thermodynamics, it was a great read, thanks for the education
Hey! thank you so much for such sweet words, I appreciate it.
I'm not expert in my field either (haha), but I love my field and the subject itself. It's just my love and passion for the subject that motivates me to write blogs. Once again, thank you for visiting :)
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.