Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Greetings to everyone! Ever wondered how scientists accurately determine the concentration of substances in solutions? Enter the Beer-Lambert Law, a brilliant tool that lets us do just that. Named after its founders, August Beer and Johann Heinrich Lambert, this law is like the magic wand of chemistry, allowing us to measure the amount of light absorbed by a substance and convert it into concentration data. In this article, we'll take a friendly tour through the Beer-Lambert Law, demystifying its science and exploring its real-world applications in the fascinating world of spectrophotometry.
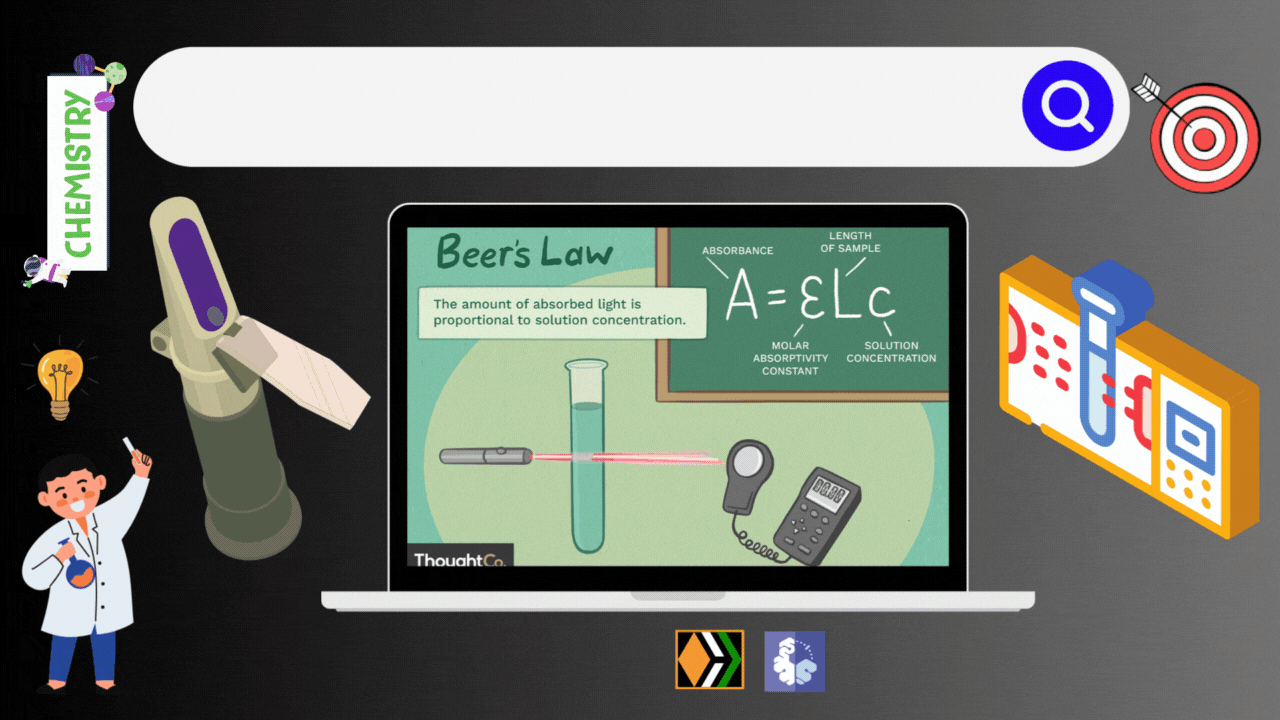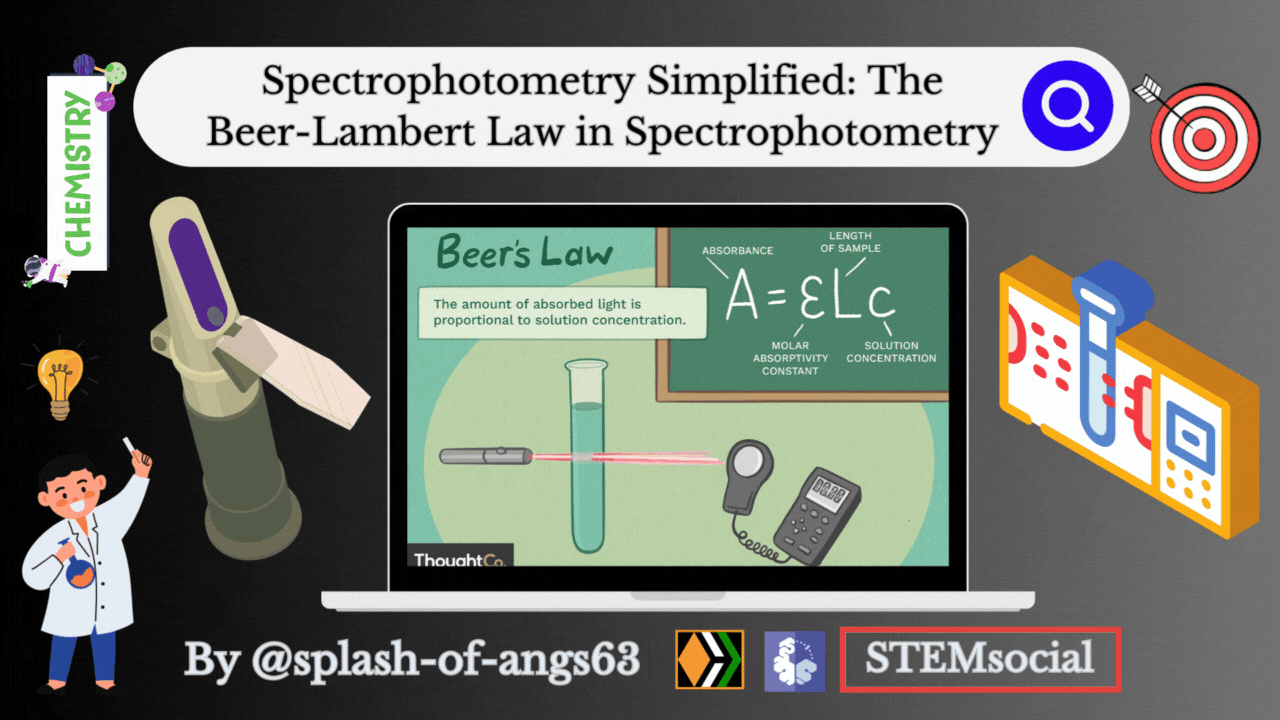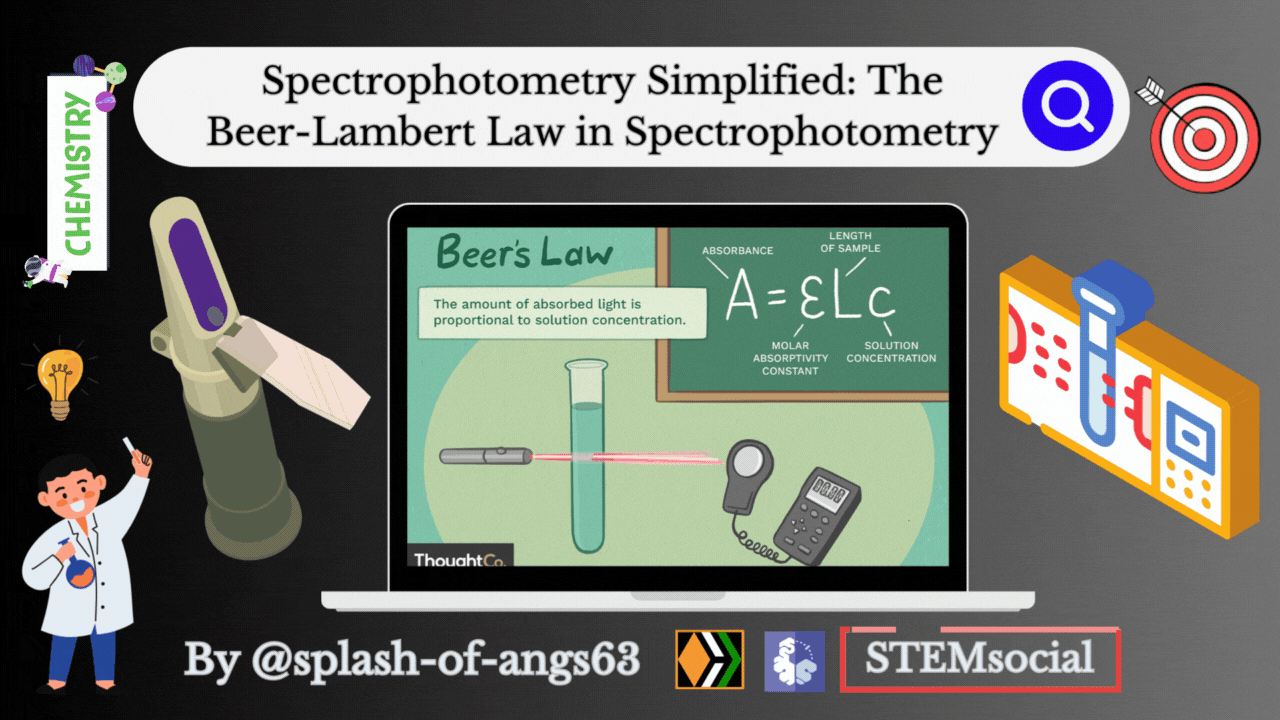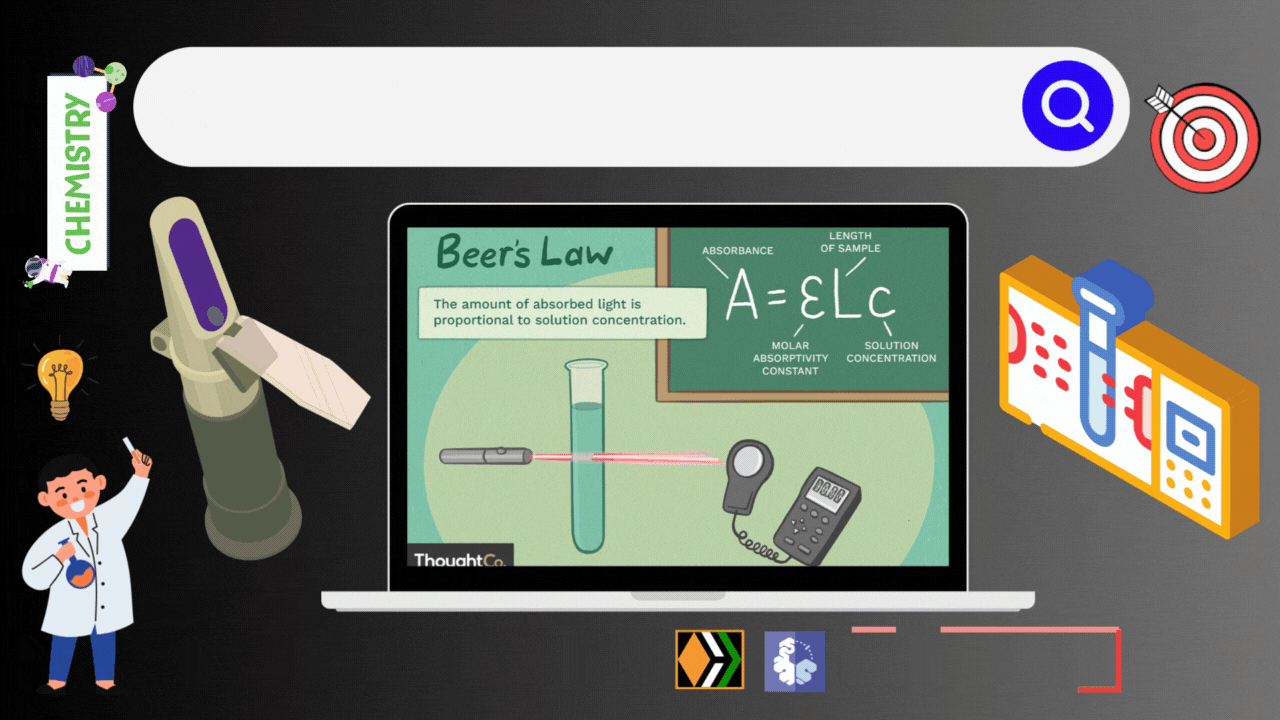

Imagine you have a special flashlight that shoots a beam of light through liquid. Now consider this liquid contains all kinds of molecules. Some of these molecules act like light magnets i.e., they absorb light, while others allow it to pass through without a fuss. Spectrophotometry is the science of measuring how much light gets absorbed and how much light comes out the other side. Thanks to the Beer-Lambert law, this measurements of light and concentration is possible.
Cracking the Code: The Beer-Lambert Law
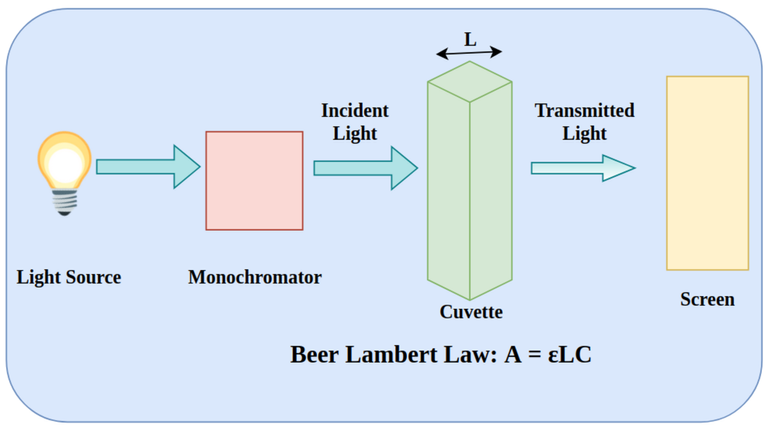
The Beer-Lambert Law gives us a mathematical way to link the concentration of a substance in a solution to the amount of light it absorbs. It's like a recipe for calculating concentration. The Beer-Lambert Law (also called Beer’s Law) is a relationship between the attenuation of light through a substance and the properties of that substance. In this article, the definitions of transmittance and absorbance of light by a substance are first introduced followed by an explanation of the Beer-Lambert Law.
The Beer-Lambert law is frequently used for absorption and transmission measurements of samples and can be used to determine the concentration of samples. In absorbance measurement, light passes through a cuvette containing the sample. The intensity of the light after passing the cuvette is compared to the light before passing through the cuvette. The size of the cuvette determines the path length (l). The wider the cuvette, the more light sample passes through and the lower the transmission.

What are Transmittance and Absorbance
Consider monochromatic light transmitted through a solution; with an incident intensity of I0 and a transmitted intensity of I.
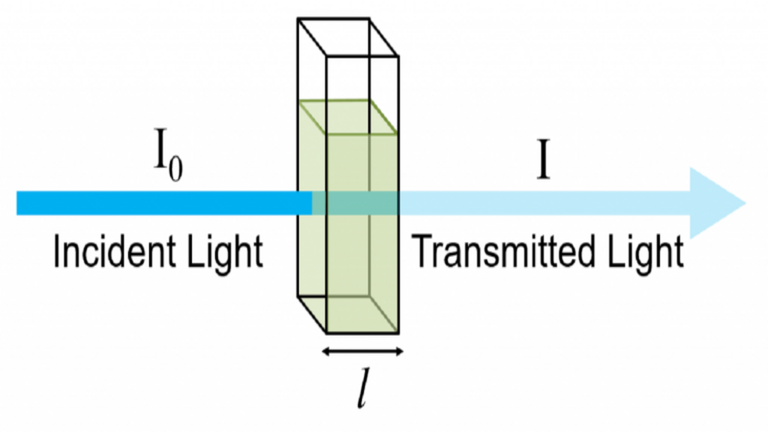
The transmittance, T, of the solution is defined as the ratio of the transmitted intensity, I, over the incident intensity, I0 and takes values between 0 and 1.
T = I/I0
However, it is more commonly expressed as a percentage transmittance;
T(%) = 100 I/I0
The absorbance, A, of the solution is related to the transmittance and incident and transmitted intensities through the following relations:
A = -log10 T
A = log10 I0/I
The absorbance has a logarithmic relationship to the transmittance; with an absorbance of 0 corresponding to a transmittance of 100% and an absorbance of 1 corresponding to 10% transmittance.

Mathematical Formulation of Beer-Lambert Law
When a beam of monochromatic radiation of a suitable frequency passes through a solution, it is absorbed by the solution. As a result the intensity of the light when it finally emerges from the solution, is considerably reduced. If I0 is the intensity of the incident beam and It is the intensity of the transmitted beam then the intensity of the light absorbed, Ia is given by,
Ia = I0 - It
The probability that the photons of a beam of intensity I will be absorbed by the sample is directly proportional to the concentration and thickness of the absorbing solution. Mathematically,
dI/I = -αcdx
where dI is the change in intensity produced by the absorption of radiation on passing through a thickness dx of the solution of concentration c and α is a proportionality constant. The negative sign shows that there is a reduction in intensity. On integrating the equation between limits I = I0 to I at x = 0 to l , we get,
ln(I/I0) = 2.303 log (I/I0) = -αlc
On putting α/2.303 = ε and defining log(I0/I) as absorbance, A of the solution, we get,
A = log(I0/I) = εlc
This is the Beer-Lambert equation.
ε is called the absorption coefficient or extinction coefficient of the absorbing medium. It is characteristic of the solute and depends upon the nature of solvent, temperature and the wave length of the radiation employed. If the concentration is exprerssed in terms of mol dm-3 and the path length l in cm, then ε is referred as molar absorption coefficient or the molar extinction coefficient.

Beer-Lambert-Law Graph
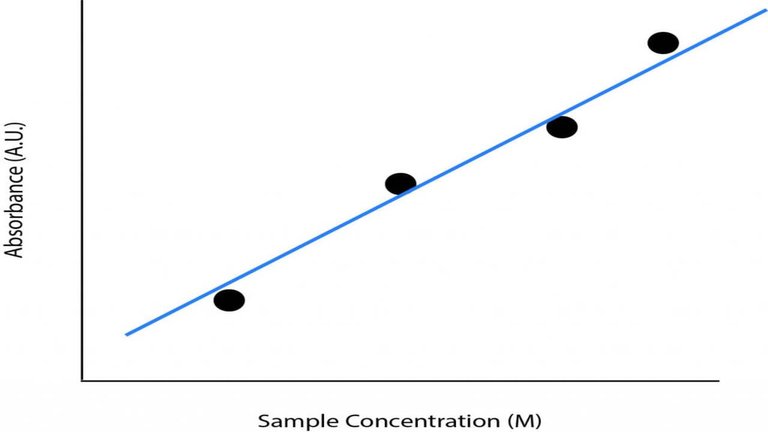
A graph showing the Beer-Lambert law will be linear and positively correlated. The x-axis will have units of concentration and the y-axis will have units of absorbance. This means that the other two variables in the equations (molar extinction coefficient and path length) remain constant. As the concentration increases, the absorbance also increases. This pattern makes sense because if the concentration increases, there are more molecules present to absorb light and cause an increase in absorption.
Below is a graph similar to the plots you will see in the Beer-Lambert Law. Various concentrations were measured. Then place a line at these points. The slope of the line is the path length multiplied by the molar extinction coefficient. If the path length is known, the molar extinction coefficient can be easily determined. The molar extinction coefficient is the slope of the line divided by the path length.

Applications of Beer-Lambert Law
The Beer-Lambert law is commonly used for determining the concentration of a sample of unknown concentration, important for experiments such as the Iodine Clock Reaction.
Lab Marvel: In the lab, scientists use the Beer-Lambert Law to find out how much of a substance is in a solution. It's like being a detective, solving the mystery of "What's in this liquid?"
Chemistry Adventures: Chemists and biochemists rely on this law to study all sorts of things—like measuring the amount of a drug in your bloodstream, investigating how enzymes work, or checking the concentration of DNA.
Eco Guardians: Environmental scientists use it to measure pollution levels in the air, water, and soil, helping us keep our planet healthy.
Quality Check: In industries like pharmaceuticals and food production, the Beer-Lambert Law ensures that medicines are potent, and our food is pure. It's like the quality control superhero of manufacturing.

Limitations of Beer-Lambert Law
The law tends to become inaccurate at high concentrations. This is due to a combination of different factors. The refractive index of the solution may deviate. There are saturation and aggregation effects possible due to the molecule of interest interacting with each other (not just solvent as is the situation at low concentrations). An excellent way to test the limitations of the Beer-Lambert Law is to make a plot of concentration versus absorption at increasingly high concentrations for a sample. The plot should be linear, but at high concentrations will stop being linear. At this point, high concentrations are causing the law to be inaccurate.
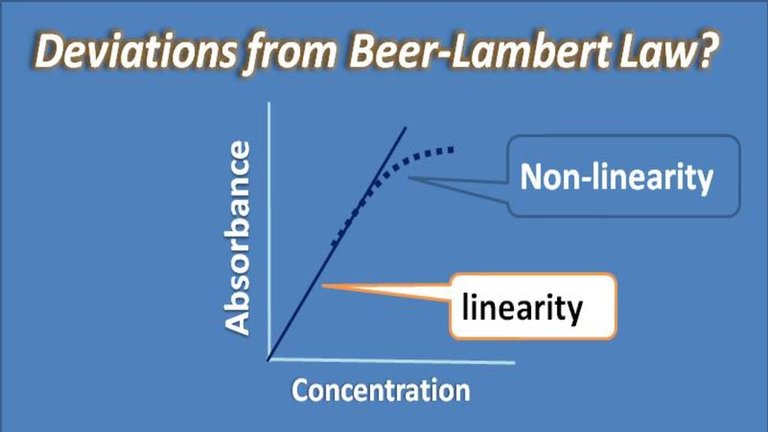
The law is not obeyed if the radiation used is not monochromatic. The temperature of the system should not be allowed to vary to a large extent. This is because the increase in temperature has a bathochromic effect on ions in solution, i.e., the absorption bands shift towards longer wavelength.
Let us now understand the uses of the law through an example.
Example: A monochromatic radiation is incident on a solution of 0.05 molar concentration of an absorbing substance. The intensity of the radiation is reduced to one-fourth of the initial value after passing through 10 cm length of the solution. Calculate the molar extinction coefficient of the solution.
Solution:
According to Beer-Lambert Law,
log (I0/I) = εlc
In this case, I/I0 = 0.25 = 25%
i.e., I0/I = 100/25
log(100/25) = ε.10cm.0.05mol dm-3
ε = 1.204 dm3 mol-1 cm-1

Conclusive thoughts
The Beer-Lambert Law, though it may sound complex, is like a trusty calculator for scientists. It allows us to uncover hidden secrets about the concentration of substances in solutions, paving the way for discoveries in medicine, environmental science, and much more. So, the next time you see a spectrophotometer in action, remember the magic of the Beer-Lambert Law, helping us make sense of the invisible world of light and molecules.
Until we meet again :)

Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
Unveiling The Control Of Chemistry: How Hormones Dictate Our Mood |ChemFam #57|
Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
PS The thumbnail image is being created by me using canva.com



This law is difficult to understand for me but it seems interesting.
bhai kahan Science padhare ho ab. Pehle achaa lagta tha ye sab.
!PGM
!ALIVE
!PIZZA
@splash-of-angs63! You Are Alive so I just staked 0.1 $ALIVE to your account on behalf of @ balvinder294. (8/10)
The tip has been paid for by the We Are Alive Tribe through the earnings on @alive.chat, feel free to swing by our daily chat any time you want, plus you can win Hive Power (2x 50 HP) and Alive Power (2x 500 AP) delegations (4 weeks), and Ecency Points (4x 50 EP), in our chat every day.

BUY AND STAKE THE PGM TO SEND A LOT OF TOKENS!
The tokens that the command sends are: 0.1 PGM-0.1 LVL-0.1 THGAMING-0.05 DEC-15 SBT-1 STARBITS-[0.00000001 BTC (SWAP.BTC) only if you have 2500 PGM in stake or more ]
5000 PGM IN STAKE = 2x rewards!
Discord
Support the curation account @ pgm-curator with a delegation 10 HP - 50 HP - 100 HP - 500 HP - 1000 HP
Get potential votes from @ pgm-curator by paying in PGM, here is a guide
I'm a bot, if you want a hand ask @ zottone444
Avi toh research chl rha hei bade bhai. Waqt nikal ke post v kr deta hoon. Haha
Ohh phir thik h. Kro continue.
$PIZZA slices delivered:
@balvinder294(5/5) tipped @splash-of-angs63
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.
Yay! 🤗
Your content has been boosted with Ecency Points, by @splash-of-angs63.
Use Ecency daily to boost your growth on platform!
Support Ecency
Vote for new Proposal
Delegate HP and earn more
(This post was manually curated by me).
divider made by irisworld
Congratulations @splash-of-angs63!
You raised your level and are now a Minnow!
Check out our last posts: