Hapticity and The Eighteen Electron Rule |ChemFam #36|
Greetings to everyone! In my previous post, we have discussed the basics of organometallic chemistry. Today, we shall be studying the topic further and will cover two most important topics in the organometallics i.e., hapticity and the 18 electron rule.

Hapticity
A single organic ligand may interact with a central metal atom using one or more of its atoms simultaneously. The number of atoms in a ligand attached to the metal atom is denoted by the prefix η (the Greek letter eta) followed by a superscript indicating the number of ligand atoms attached to the metal atom. This is called hapticity. Most ligands attached through one atom only, therefore they are called monohapto (η1) ligands. Cyclopentadienyl ligand, C5H5- or Cp, for example can attach to metal atom through one, three or five carbon atoms. Therefore, it may acts as mono (η1)-, tri (η3)- or pentahapto (η5)- ligand.
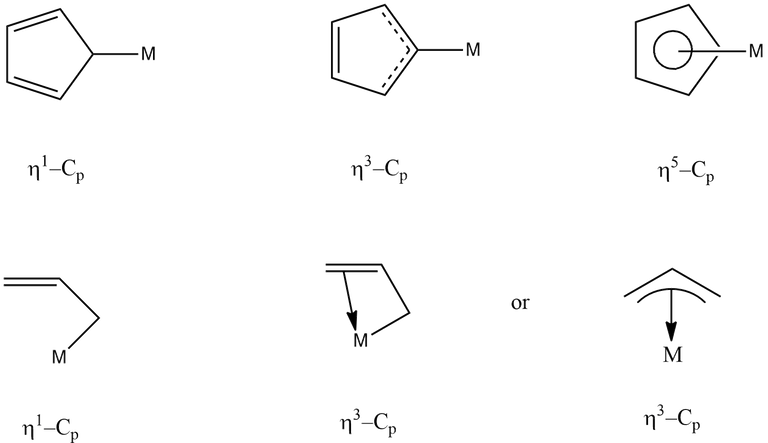
The term hapto comes from Greek word haptein meaning to fasten. Therefore, pentahapto means fastened in five places.
The hapticity of various ligands are shown below-
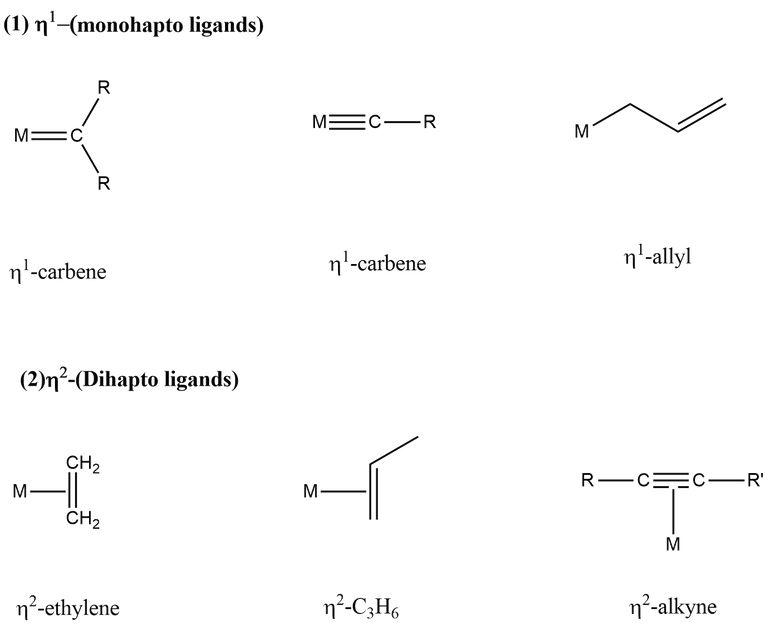

The ligand which can change its hapticity is called a fluxional ligand. If hapticity of a ligand is not given, then the ligand has to be assume in its highest hapticity, e.g., Cp → η5, allyl → η3.
Eighteen Electron Rule
The main group elements such as lead uses its four valence electrons for the formation of four σ bonds and as a result a stable compound such as PbEt4 is obtained. In such compounds, the total number of valence electrons becomes equal to 8,i.e., these compounds obey octet rule. On the other hand PbEt3 contains only six valence electrons and therefore does not obey octet rule and is unstable. However there are some complexes which do not obey the octet rule and are unstable.
In general organometallic compounds of transition metals are formed with metals that are in low oxidation states (-1, 0, and +1). According to Sidwick's effective atomic number (EAN) rule, the EAN of a metal is equal to the sum of electrons on a metal plus the electrons donated by the ligands and EAN is equal to the atomic number of next noble gas.
An An alternate and more general rule is the 18 electron rule. According to this rule, in an organometallic compounds and other complexes the sum of valence shell electrons of transition metal or metal ion and electrons donated by the ligands is equal to 18. It provides a closed and stable valence shell configuration (n-1)d10ns2np6. In transition metals the electrons of (n-1)d and ns electrons are considered to be valence electrons. The complex which obey the EAN or 18 electron rule are considered to be stable. The EAN and 18 electron rules are similar. The 18 electron rule is a rule of thumb and is more advantageous than EAN because there is no need to remember the atomic number of the noble gases. However there are some exceptions which obey neither EAN nor 18 electron rule. The mononuclear compounds in which the number of total valence electrons is odd never obey either EAN or 18 electron rule.
The first row transition metal carbonyls mostly obey the 18 electron rule. Each metal contributes the same number of electrons as its group number and CO ligand contributes two electrons, π- back bonding makes no difference to the electron count for the metal. In case of transition metals with odd electrons, even number of electron 18 is never obtained by adding two electron ligands such as CO. In each case the system resolves the problem in a different way. The complex V(CO)6 has 17 valence electron and easily reduced to the 18 electron [V(CO)6]- anion. In V(CO)6, the vanadium is too small to permit a seventh coordination site because there would be strong steric hinderance. Therefore, no metal-metal bond dimer is formed which would give an 18 electron configuration.
Unlike V(CO)6, the Mn(CO)5 species which is also a 17 electron species, does dimerise with the formation of M-M bond. This is due to the reason that the coordination of the 5 coordinate species has more space available to make the M-M bond. Each metal atom in the dimer obeys 18 electron rule because the unpaired electron in each 5 coordinate species is shared with the other in forming the M-M bond as much as the 7 electron methyl radical dimerizes to give the 8 electron compound, C2H6.
Let us now, apply the 18 electron rule to some of the organometallic compounds by neutral method.
(1) HMn(CO)5
electrons contributed by the metal atom = 7 e-
electrons contributed by 5 CO ligands = (5x2) = 10 e-
electron contributed by H = 1 e-
Total electron count (TEC) = 18 e-
(2) (η5- C5H5)2Fe
electrons contributed by the metal atom = 8 e-
electrons contributed by 2 Cp ligands = (2x5) = 10 e-
Total electron count (TEC) = 18 e-
(3) (η5- C5H5)Fe(CO)2Cl
electrons contributed by the metal atom = 8 e-
electrons contributed by Cp ligand = 5 e-
electron contributed by 2 CO ligands = 4 e-
electron contributed by 1 Cl = 1 e-
Total electron count (TEC) = 18 e-
(4) CH3Mn(CO)5
electrons contributed by the metal atom = 7 e-
electrons contributed by 5 CO ligands = (5x2) = 10 e-
electron contributed by CH3 = 1 e-
Total electron count (TEC) = 18 e-
I hope I have covered all the basics of the two most important topics. We shall meet again, happy learning!
Organometallic Chemistry (Evans)
Organometallic compounds- An overview
An Introduction To Organometallic Chemistry |ChemFam #35|
Applications of Zeolites: The 3D Molecular Sieves |ChemFam #34|
Properties of Zeolites: The 3D Molecular Sieves |ChemFam #33|
Zeolites: The 3D Molecular Sieves |ChemFam #32|
The Beauty of Eucalyptus Tree |ChemFam #31|
The Accidental Cure for Cancer: Cisplatin |ChemFam #29|
Acceptorless Dehydrogenation and Related Transformations |ChemFam #28|
Thermophysical Properties of Natural Gas-I |ChemFam #27|
Sources and Process Overview of Natural Gas |ChemFam #26|
PS The thumbnail image is being created by me using canva.com


Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited.