Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Greetings to everyone! With the rapid development of the economy, the demand for lithium resources is increasing. Conventional processes such as precipitation usually take 1 to 2 years and are weather dependent. In addition, electrochemical lithium recovery (ELR) has received great attention as a green chemistry method. Here, we shall be discussing about the recent advances on the electrochemical lithium extraction technology from saltwater/seawater and electrode materials for lithium-ion batteries. Finally, we look forward to the future opportunities and challenges of electrochemical lithium extraction. I shall be trying to give an in-depth discussion of the electrochemical lithium extraction system and electrochemical lithium extraction from brine/seawater, with special attention to the electrodes and system that provides important guidelines for electrochemical design.

Lithium-ion batteries are rechargeable power sources used in smartphones, laptops, electric vehicles, and renewable energy storage systems. They consist of two main electrode materials: the cathode and the anode. The cathode typically contains lithium compounds, such as lithium cobalt oxide (LiCoO2), lithium iron phosphate (LiFePO4), and lithium manganese oxide (LiMn2O4). On the other hand, the anode is usually made of graphite or lithium titanate (Li4Ti5O12).

The rapid growth of the economy and the increasing demand for raw materials require the exploration of mineral resources in order to meet and accelerate production needs. Among some precious metals, lithium is an important element of economy with the lightest metal density (0.53 g/cm3) and the most negative redox potential (-3.04 V) commonly used in portable electronic devices, vehicle energy and energy storage. In recent years, with the development of electric vehicles, many lithium-ion batteries have been used in electric vehicles and the amount of lithium used in batteries has increased, accounting for 65% of lithium-ion end market.
Accordingly, it is particularly important to find an efficient method for the extraction of lithium. Currently, lithium is mainly obtained from hard rock and lithium solutions including natural gas, seawater, and spent lithium electrolytes. Lithium extraction from hard rock is energy intensive, expensive and causes high pollution. In addition, salt water / sea water is rich in lithium, which accounts for about 80% of all known reserves. Moreover, the total stock of lithium in the world's oceans, is about 230 billion tons, so the oceans have received great attention.
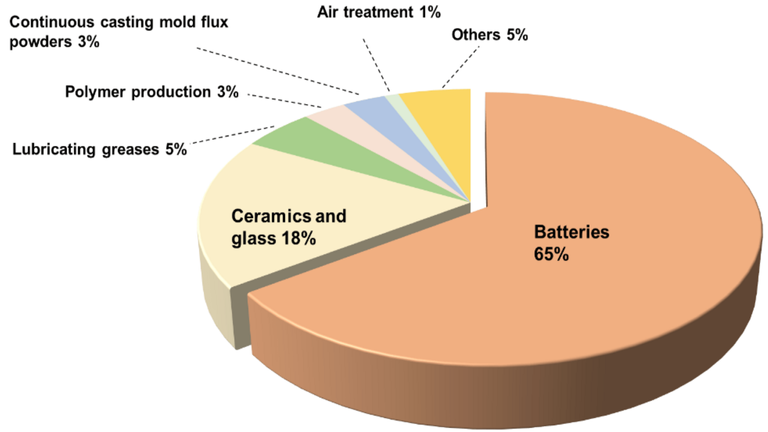
Therefore, improving the economic and environmental conditions for lithium recovery from brine/seawater will be a positive aspect of lithium recovery in the future.

Currently, many methods have been reported for lithium recovery from brine/seawater such as precipitation, adsorption, extraction, ion exchange and electrodialysis. Among them, the precipitation method is a mature and reliable method, and it is also the oldest method in the industry to extract lithium from salt lakes. However this process requires a pretreatment that takes a long time to complete and is affected by weather conditions. Moreover the addition of lime to separate lithium and magnesium adds cost and creates waste, which puts a lot of pressure on the environment. Studies have clearly shown that precipitation can be used for separation when the ratio of magnesium to lithium is greater than 6. However, chemical precipitation is not applicable as most brines have a low magnesium/lithium ratio. Also, Mg and Li have similar radii and similar properties. It is necessary to develop a new way to obtain lithium.
In recent years, electrochemical lithium extraction has attracted great attention from researchers as a method with high efficiency, environmental protection, easy operation and no environmental pollution. In fact, the electrochemical extraction method of lithium mainly achieves the embedding and deintercalation of Li+ ion in electrode material by controlling the potential so as to achieve the purpose of lithium extraction from brine/seawater.

Electrochemical Lithium-Recovery Systems
To achieve efficient extraction of Li many Li recovery electrochemical systems have been reported. These includes water-splitting battery system, asymmetric battery systems, hybrid supercapacitor systems and symmetrical rocking chair battery-liked systems. In this article, we shall be studying the water splitting battery and the asymmetric battery systems.
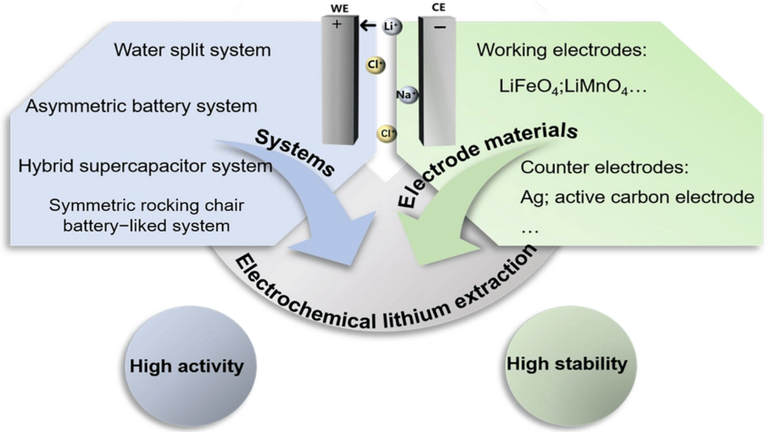
Water Splitting Battery System
The water splitting battery system is the oldest electrochemical lithium recovery (ELR) system consisting of a working electrode (WE) and a counter electrode (CE), where the capture of Li+ and water splitting process occurs simultaneously. In general, this CE not only supports the design of the closed circuits, but also guarantees the electrical neutrality of the system. Kanoh et al. reported the λ-MnO2/Pt system that can achieve Li intercalation through the exchange of λ-MnO2 and LiMnO2. Despite the high selectivity of lithium ions, the water splitting that occurs at the platinum electrode, (as shown by the reaction below) increased energy and leads to lower lithium extraction efficiency. On the other hand, the pH status should be maintained during Li recovery.
1/2 𝐻2𝑂→1/4 𝑂2 + 𝑒− + 𝐻+
Asymmetric-Battery System
The asymmetric battery is considered to be an effective lithium extraction system with stable acidity and alkalinity. For example, Li et al., for a rechargeable battery, coupled the λ-MnO2 electrodes with silver that exhibited good reversibility. Generally, this type of λ
-MnO2-Ag battery can achieve Li-ion recovery without being affected by other cations in salt water, which is the reason for the Li-ion sieve property originating from the crystal structure of the electrode. However, the high cost and poor recovery of silver electrodes limit their wide range of applications and therefore suitable alternatives have attracted the attention of many.
Polyaniline as a polymer material and has been recognized as a good electronic material for lithium recovery because of its excellent reversibility as well as its chemical and environmental stability. For example, Zhao et al. constructed a high-performance, low-cost and non-polluting polyaniline/LixMn2O4 battery that can extract LiCl with excellent lithium ion selectivity and excellent chloride ion capture ability. Generally, this reaction occurs in the PANI (polyaniline) based electrode and follows p-doping/de-doping as shown by the equation below. In addition, open-frame analogs of Prussian blue are the best substitutes for silver because of their charge retention capability.
𝑃𝐴𝑁𝐼 + 𝐶𝑙- − 𝑒- ⇆ 𝑃𝐴𝑁𝐼 + 𝐶𝑙−

La Mantia et al. replcaed the Fe3+ with Ni2+ to form nickel hexacyanoferrate (NiHCF), which is used as CE thanks to its selectivity for K+ and Na+. Overall, this environmentally friendly NiHCF could achieve low energy consumption and inexpensive bulk synthesis. Similarly, this asymmetric battery system can selectively recover Li+ using λ-MnO2 electrodes.

Advantages of Extraction of Li from Electrode Material
This method offers several advantages which includes :
Conservation of resources: Lithium recovery from used batteries can reduce the need for new lithium mining, thereby conserving natural resources and reducing the environmental impact of mining operations.
Reducing emissions: Recycling lithium-ion batteries and removing lithium in environmentally friendly ways can help reduce carbon monoxide emissions and other harmful effects associated with lithium extraction.
Cost-effective: Recycling lithium-ion batteries can provide significant benefits of lithium compared to the mining process.
Coclusive thoughts
The increasing demand for lithium due to the popularity of lithium-ion batteries in many applications requires a sustainable extraction method. Lithium extraction using Li-ion battery electrode materials offers a practical solution based on circular economy and money saving principles. By using environmentally friendly technology, we are not only meeting the lithium demand, but also paving the way for a greener and sustainable future. Government, industry and consumers must work together to facilitate the recycling and reuse of lithium-ion batteries to ensure the long-term benefits of this resource.
We shall meet again :)
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
SCRAP Giveaway | Terracore | Draw #10 |
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
SCRAP Giveaway | Terracore | Draw #5 |
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
What are Antimicrobials and Antimicrobial Drugs? |ChemFam #44|
Therapeutic Action of Different Classes of Drugs |ChemFam #43|
Introduction to Drugs and Drug-Target Interaction |ChemFam #42|
Scientists Analyze a Single Atom With X-Rays For The First Time |ChemFam #41|
Can We Slow Down Aging? |ChemFam #40|
Studying The Cluster Compounds: The LNCC |ChemFam #39|
PS The thumbnail image is being created by me using canva.com by using template image from mdpi


Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next payout target is 1000 HP.
The unit is Hive Power equivalent because post and comment rewards can be split into HP and HBD
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out our last posts:
Hi, bro next-level information regarding lithium and electron*
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.