Basics to Quantum Chemistry: Operators, Functions and Properties of Operators |ChemFam #75|
Greetings to everyone! Classical mechanics as formulated by Sir Isaac Newton is obeyed by macroscopic particles such as planets and rigid bodies. However, since the microscopic particles such as electrons, protons, atoms and molecules show wave-particle dual nature, and hence they do not obey Newtonian dynamics. They however obey Quantum Mechanics (or wave mechanics), a key feature of which is the quantization of energy and angular momentum. So, yes, I am going to start discussing quantum chemistry now. But since quantum chemistry is a vast and conceptual topic, it is better to start from some basic mathematics and mathematical tools used in quantum chemistry.
Function
Basically a function is a rule that relates two or more variables. This mathematical constructs plays a pivotal role in representing the wavefunctions of electrons and the properties of molecular systems.
Suppose, z=x2+y2, then z is a function of the variables x and y.
mathematically, z = f(x,y)
From previous studies on thermodynamics, we have learnt that volume, V is a function of pressure, P and temperature, T.
So, we can write, V = f(P,T)
This simply means that by varying values of pressure, P and/or temperature, T; value of V also varies. Thus, V is a function of T and P.
In quantum chemistry, the wavefunction is a central concept, representing the quantum state of a system. The wavefunction provides information about the distribution of electrons in a molecular system and serves as the foundation for calculating various properties such as energy, geometry, and reactivity. The wavefunction, denoted as Ψ, is often expressed as a mathematical function of the spatial coordinates of electrons. We shall discuss wavefunction in a separate blog, giving a rough idea about wave function should be sufficient for the discussions that we shall be doing for today.
Operator
Operators are pre-defined process or operation that changes one function to another function or constant entity. An operator is denoted by a 'hat' or a 'cap' (^).
Let us understand it by taking an example.
Just like we need minimum of two values for doing addition, subtraction, multiplication or division, likewise we need a function with an operator to draw physical significance. In the example above, we found the first derivative by doing operation on a function f(x).
Properties of operators
- Operator is meaningless without a function.
- Operators are directional in nature. i.e., they can be applied from left to right, but they will lose sense if the direction is reversed, say from right to left.
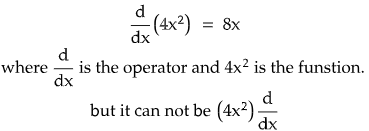 -
- - The nth power of operator means the operator will be operated n number of times.
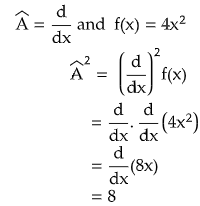
- In quantum mechanics every operator is linear.
For an operator to be linear,
(a)  {c f(x)} = c  f(x), where c is a constant.
Let us see by taking an example.
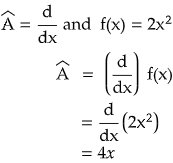
Therefore, d/dx is a linear operator. Similarly integration is also a linear operator. Can you tell me whether square root is a linear operator or not?
(b) Â {f(x)+g(x) = Â {f(x)} + Â {g(x)}
- For every observable in classical mechanics, there is a operator in quantum mechanics. An observable is a variable that changes with the motion such as position, momentum, energy etc.
Let us see some of the important operators and their operation which will be frequently used in our studies.
Table 1) Classical observable and its quantum operator and operation
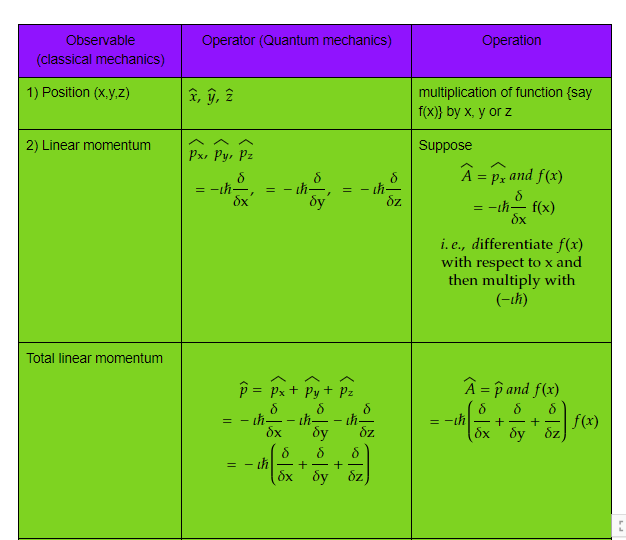
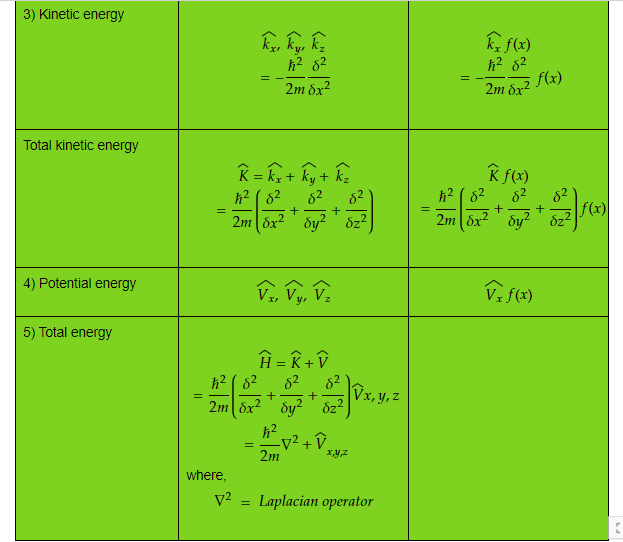
Complete table can be found at, https://www.mathcha.io/editor/nKoEvu7QSeWtrL1l4ws6YN6Glcr90P8GHEOZkwQ
(All rights reserved to me)
Angular momentum operator in quantum chemistry
As an extension of the classical concept of angular momentum, the quantum mechanical counterpart introduces unique features that govern the dance of electrons around nuclei. The angular momentum operator, often denoted as L̂ is a vector operator in quantum mechanics. Its components, L̂x, L̂y and L̂z, represent the projections of angular momentum onto the x, y, and z axes, respectively.
The three-dimensional angular momentum is a vector quantity and for a point particle it is classically represented as a pseudovector r × p, the cross product of the particle's position vector r (relative to some origin) and its momentum vector; p.
We can deduce the angular momentum operator for x, y and z directions based on the linear momentum operators we have learnt today.
Now, suppose r is a position vector having all the three dimensions.
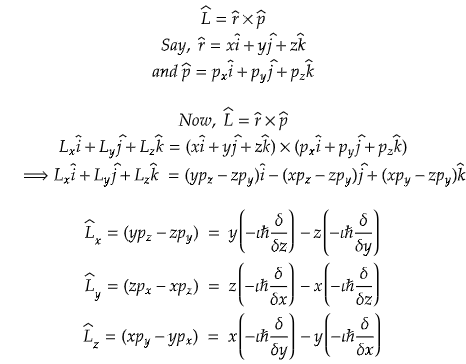
Problem) The angular momentum operator L̂y is?
Solution
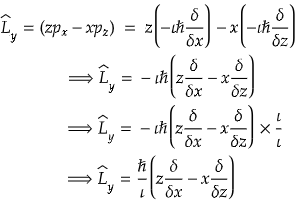
What we learnt?
We were able to identify, define and understand what a function is! Functions are indispensable tools that allow scientists to model and understand the behavior of electrons and molecules. Whether describing the spatial distribution of electrons, calculating energy, or characterizing molecular orbitals, functions are the backbone of quantum chemical methods.
We learnt what a operator is in quantum mechanics and how it is derived from classical mechanics. We discussed the various properties of operator.
The angular momentum operator in quantum chemistry serves as a fundamental tool for understanding the behavior of electrons in atoms and molecules.
Software used:
The mathematical equations are prepared using mathcha.io editor.

A Comprehensive Study of Euler's Reciprocal Rule in Thermodynamics |ChemFam #73|
A Deep Dive into Nutrition Essentials: Your Path to a Healthier, Happier You |ChemFam #72|
Decoding Liver Function Tests through Chemistry |ChemFam #71|
Understanding the Dynamic Roles of Metalloenzymes and Metal-Activated Enzymes |ChemFam #70|
Cracking the Thermal Code: Differential Thermal Analysis in Modern Research |ChemFam #69|
Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
The Silent Revolution: How Polymers are Shaping Our World? |ChemFam #67|
Beyond the Bin: The Many Faces of Plastic Management |ChemFam #66|
Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
Unveiling The Control Of Chemistry: How Hormones Dictate Our Mood |ChemFam #57|
Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
PS The thumbnail image is being created by me using canva.com


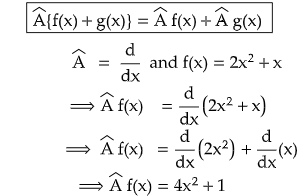

Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 15000 upvotes.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPThis post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited..
This post received an extra 2.14% vote for delegating HP / holding IUC tokens.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.