Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
Greetings to everyone! In the world of science, there exists a vast array of analytical techniques, each serving a unique purpose and offering valuable insights into the composition and structure of matter. Among these techniques, infrared spectroscopy stands out as a powerful tool used extensively in chemistry, biology, and various other scientific disciplines. In this article, we will explore the principles, applications, and the compelling need and importance of infrared (IR) spectroscopy.
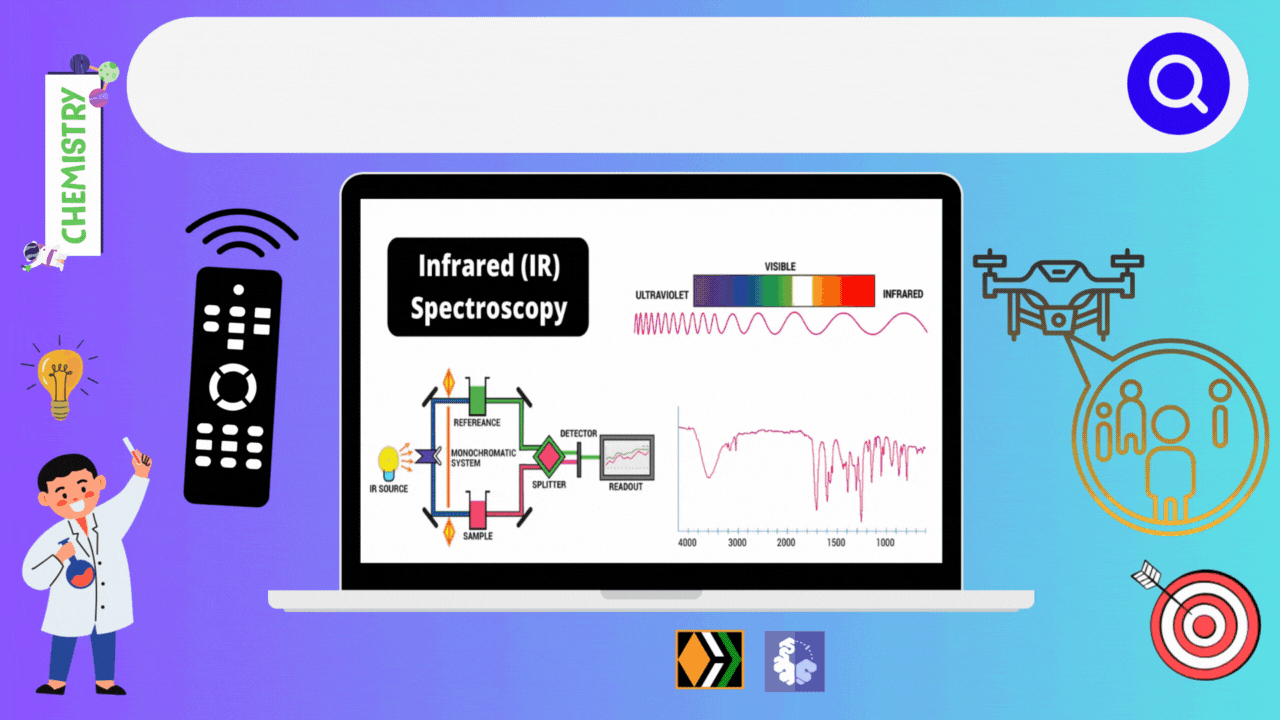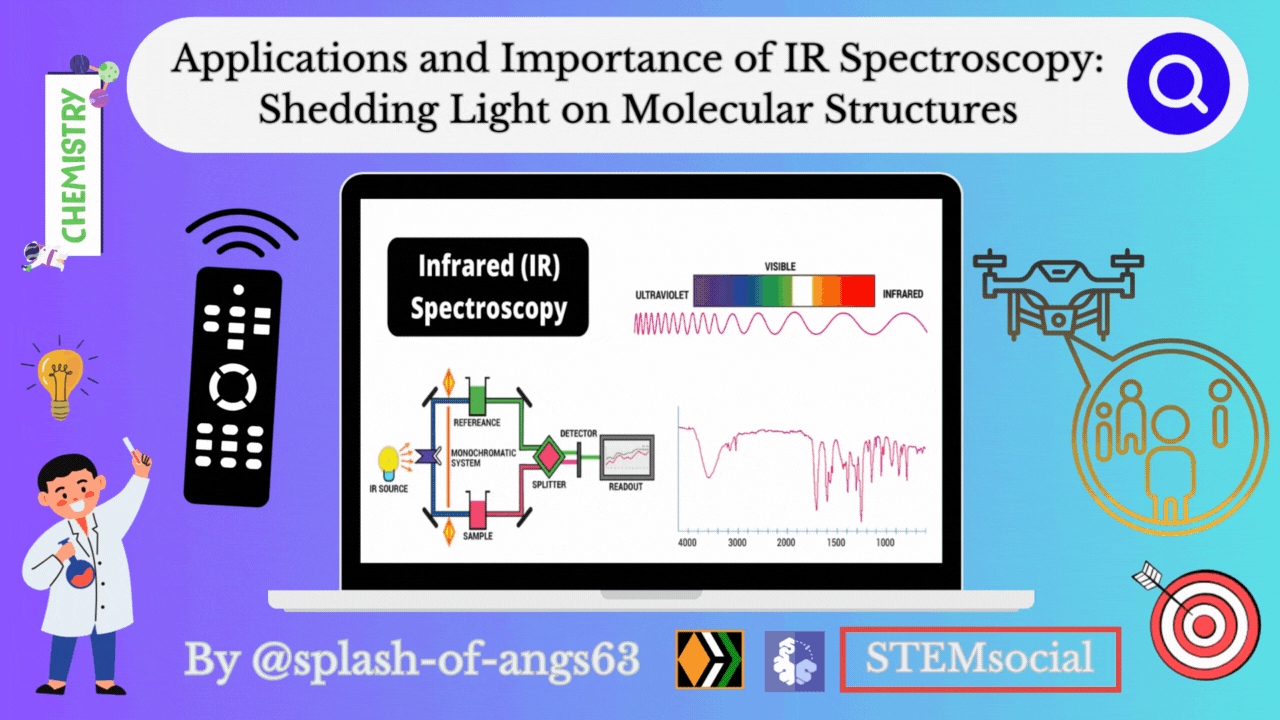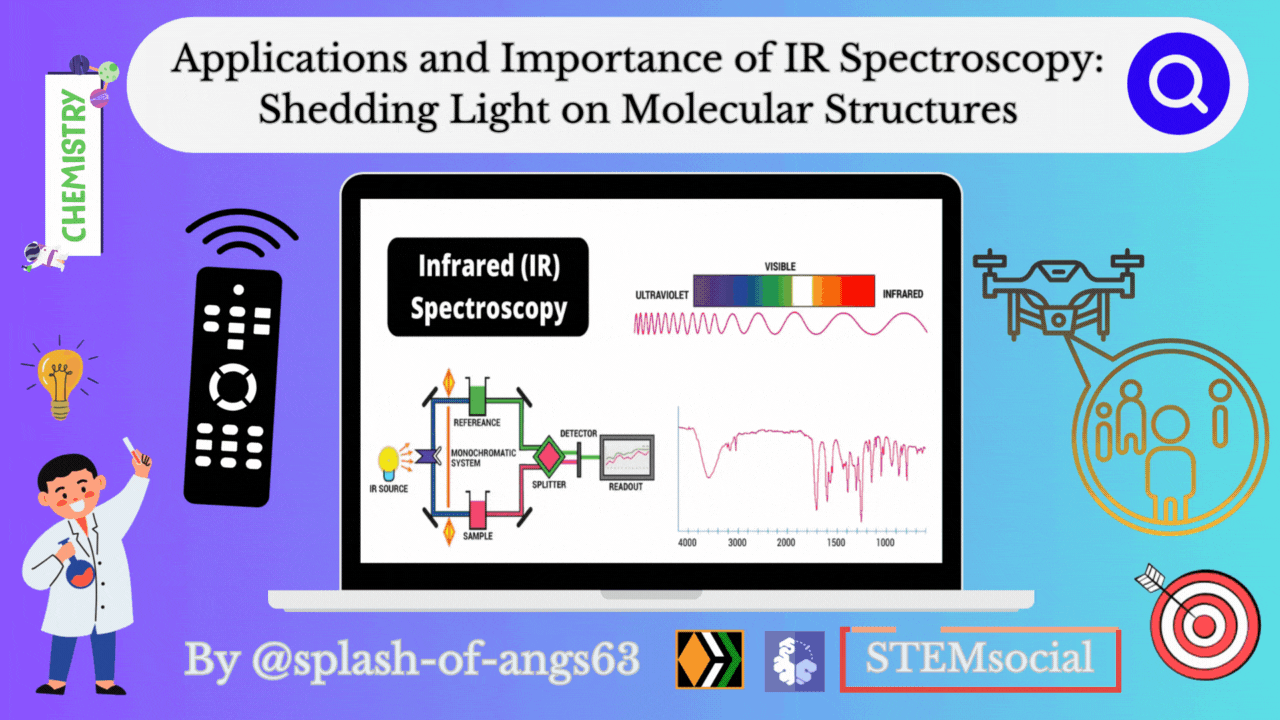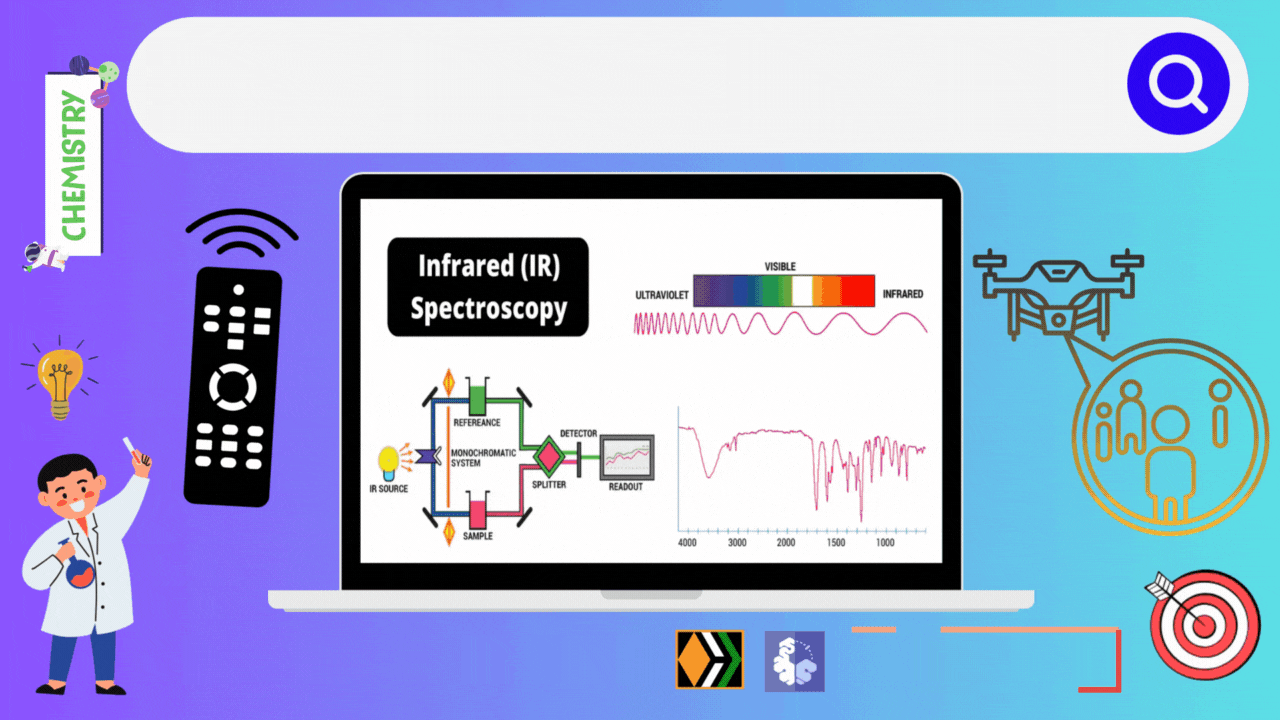
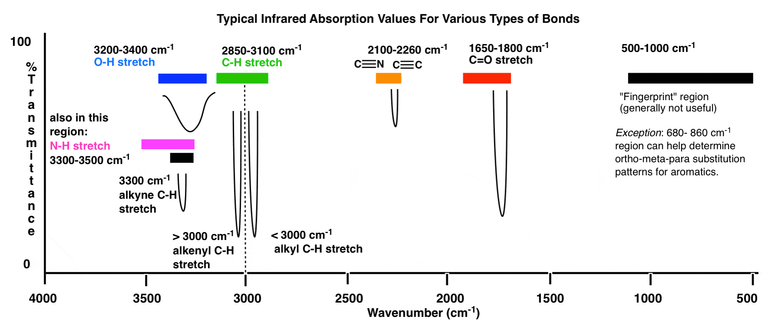
Infrared spectroscopy deals with the recording of the absorption of radiations in the infrared region of the electromagnetic spectrum. The position of a given infrared absorption is expressed in terms of wavelength in micron μ or more commonly in terms of wavenumber ṽ (cm-1) since it is directly proportional to energy.
The ordinary infrared region 2.5-15 μ (4000-667 cm- ) is of greatest practical use to organic chemists. The region 0.8-2.5 μ (12,500- 4000 cm-1) is called the near infrared and the region 15-200 μ (667-50 cm-) is called the far infrared. The absorption of infrared radiation by a molecule occurs due to quantized vibrational and rotational energy changes when it is subjected to infrared irradiation. Thus, IR spectra are often called vibrational-rotational spectra.
Theory of Infrared Spectroscopy
IR absorption spectra originate from transitions in vibrational and rotational energy Ievels within a molecule. On absorption of IR radiation, vibrational and rotational energies of the molecule are increased.
When a molecule absorbs IRradiation in the range 100-10,000 cm-1, the absorbed radiation causes transitions in its vibrational energy Ievels. These energy Ievels are also quantized, but vibratonal spectra appear as bands rather than discrete lines. The energy differences between various rotational energy Ievels of a molecule are far less than that between its vibrational energy Ievels. Thus, a single transition in vibrational energy Ievels is accompanied by a large number of transitions in rotational energy Ievels and so the vibrational spectra appear as vibrational-rotational bands instead of discrete lines. Organic chemists are mainly concemed with these vibrational-rotational bands, especially with those occurring in the region 4000-667 cm-.
Modes of Molecular Vibrations
Various atoms in a molecule may be regarded as balls of different masses and the covalent bonds between them as weightless tiny springs holding such balls together. Atoms in a molecule arenot still but they vibrate. The two types (modes) of fundamental molecular vibrations known are: (a) stretching and (b) bending vibrations (deformations).
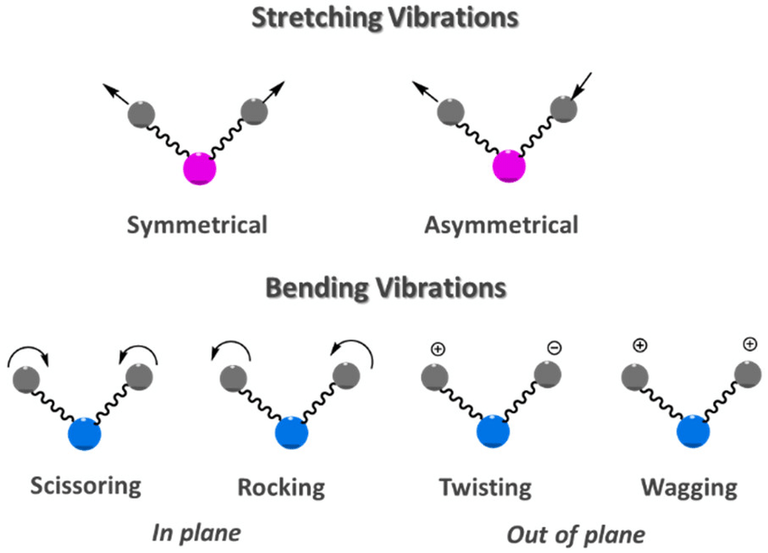
(i) Stretching Vibrations
In stretching vibrations, the distance between two atoms increases ordecreases, but the atoms remain in the same bond axis. Stretching vibrations are of two types:
- (a) Symmetrical stretching: In this mode of vibration, the movement of atoms with respect to the common (or central) atom is simultaneously in the same direction along the same bond axis.
- (b) Asymmetrical Stretching: In this vibration, one atom approaches the common atom while the other departs from it.
(ii) Bending Vibrations (Deformations)
In such vibrations, the positions of the atoms change with respect to their original bond axes. Bending vibrations are of four types:
- (a) Scissoring: In this mode of vibration, the movement of atoms is in the opposite direction with change in their bond axes as well as in the bond angle they form with the central atom.
- (b) Rocking: In this vibration, the movement of atoms takes place in the same direction with change in their bond axes.
Scissoring and rocking are in-plane bendings. - (c) Wagging: In this vibration, two atoms simultaneously move above and below the plane with respect to the common atom.
- (d) Twisting: In this mode of vibration, one of the atom moves up and the other moves down the plane with respect to the common atom.
The Importance of IR Spectroscopy
- 1. Structural Analysis
One of the primary applications of IR spectroscopy is determining the structure of organic and inorganic compounds. It's an indispensable tool for chemists, enabling them to identify unknown substances and confirm the presence of specific functional groups within a molecule. For example, in the field of drug development, IR spectroscopy plays a critical role in characterizing and confirming the structure of newly synthesized compounds.
- 2. Quality Control
Industries such as pharmaceuticals, food, and petrochemicals rely heavily on IR spectroscopy for quality control and assurance. By comparing the spectra of raw materials and finished products, manufacturers can ensure the consistency and purity of their products. Any deviations from the expected spectra can signal impurities or undesired chemical changes.
- 3. Environmental Analysis
Environmental scientists and regulators use IR spectroscopy to monitor and analyze pollutants in the atmosphere and water. By identifying the specific compounds contributing to pollution, steps can be taken to mitigate the environmental impact and safeguard human health.

Scientific Reports (Sci Rep) ISSN 2045-2322 (online)
(A) Averaged FTIR spectra and (B) averaged second derivative of the FTIR spectra of the COVID-19 positive and negative groups of the LIQUID 1 dataset.
- 4. Forensic Science
IR spectroscopy has found its place in forensic science as well. Investigators use it to analyze trace evidence, such as fibers, paints, and drugs. This aids in solving crimes, identifying substances, and linking evidence to specific individuals or locations.
- 5. Material Science
In the field of material science, researchers use IR spectroscopy to study the properties of materials like polymers, ceramics, and composites. Understanding the molecular structure and behavior of these materials is essential for developing new and improved products for various applications.
The Need for IR Spectroscopy
The need for IR spectroscopy is driven by its unique capabilities and the information it provides. Here are some reasons why this technique is essential in the scientific world:
- 1. Non-Destructive Analysis
Unlike some other analytical methods, IR spectroscopy is non-destructive. It doesn't alter or consume the sample being analyzed. This is especially valuable when working with precious or limited quantities of a substance.
- 2. Versatility
IR spectroscopy is highly versatile, accommodating a wide range of samples, from liquids and gases to solids. It can be used in various fields, from chemistry to biology to materials science, making it a versatile and widely applicable technique.
- 3. Speed and Precision
The speed at which IR spectra can be obtained is impressive, with results available in seconds. This rapid analysis is crucial in industries where quick decisions are necessary, such as pharmaceutical manufacturing.
- 4. Quantitative Analysis
In addition to qualitative analysis, IR spectroscopy can be used for quantitative analysis. It can determine the concentration of specific compounds within a mixture, making it a powerful tool for quality control and process monitoring.
- 5. Minimal Sample Preparation
Sample preparation for IR spectroscopy is relatively simple, often requiring minimal or no preparation at all. This simplifies the analytical process and reduces the chances of introducing errors.
Conclusive thoughts
Infrared spectroscopy is more than just a scientific technique; it's a cornerstone of modern research and industry. Its ability to reveal the hidden molecular architecture of matter has made it indispensable in a wide range of applications, from chemistry and materials science to environmental monitoring and forensic investigations. The need for IR spectroscopy arises from its unparalleled versatility, speed, precision and ability to provide valuable information without damaging precious samples. As science continues to advance, IR spectroscopy remains a shining beacon of insight, shedding light on the intricacies of the molecular world.
Until we meet again :)

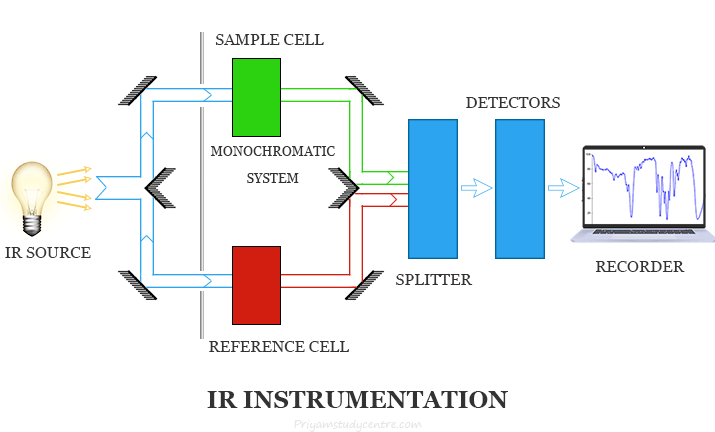
IR Spectroscopy Principle and Instrumentation
IR Spectroscopy | ACS Reagent Chemicals
The Silent Revolution: How Polymers are Shaping Our World? |ChemFam #67|
Beyond the Bin: The Many Faces of Plastic Management |ChemFam #66|
Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
Unveiling The Control Of Chemistry: How Hormones Dictate Our Mood |ChemFam #57|
Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
Hydrogen: The Simplest Atom |ChemFam #51|
Elements, Atoms and Atomic Theory |ChemFam #50|
Have You Thanked A Clod Today? |ChemFam #49|
Nuclear Energy: Will It Rise Again? |ChemFam #48|
Soaps: An Essential and Effective Cleansing Agent |ChemFam #47|
Chemicals in Food : Debunking Myths and Ensuring Safe Consumptions |ChemFam #46|
Unveiling The Secrets of Antiseptics and Disinfectants |ChemFam #45|
PS The thumbnail image is being created by me using canva.com



Congratulations @splash-of-angs63! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 250 posts.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPTo support your work, I also upvoted your post!
Check out our last posts:
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.