A Comprehensive Study of Euler's Reciprocal Rule in Thermodynamics |ChemFam #73|
Greetings to everyone! In chemical thermodynamics, the concept of exact differentials plays a crucial role, particularly when analyzing the behavior of ideal gases. An exact differential is a mathematical concept that relates to a function whose differential is independent of the path taken between two points in a system. In the context of chemical thermodynamics, one commonly encountered equation where the concept of exact differentials is applicable is the ideal gas equation.
The ideal gas equation is given by:
PV=nRT
where:
- P is the pressure of the gas,
- V is the volume of the gas,
- n is the number of moles of the gas,
- R is the ideal gas constant, and
- T is the temperature of the gas in Kelvin.
To examine the exact differentials associated with the ideal gas equation, let's consider the differential form of the ideal gas equation:
If Z is a function of the variable x and y,
then we can write as Z = f(x,y)
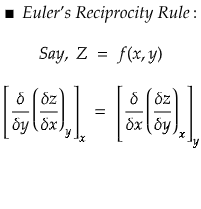 then Z will be termed as an exact differential entity.
then Z will be termed as an exact differential entity.
Ok! So we understood how to know if an entity is an exact differential or not. If both the sides are equal, then it is an exact differential. Let's now take an actual example to verify the validity of the theory.
example 1) if Z = 8x2y2, Find out whther Z is an exact differential or not?
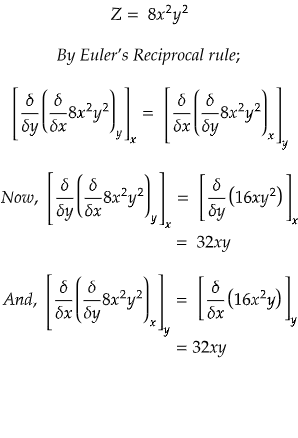
Since, both RHS and LHS are equal. Hence, Z is an exact differential.
Great! We have learnt how exactly we can apply the rule to verify if the entity is an exact differential or not!
Now, Let's apply the rule to thermodynamics.
Let's see how this rule can be applied to the ideal gas equation to verify the exact diffeential property of the state variables or state functions i.e., P, V and T.
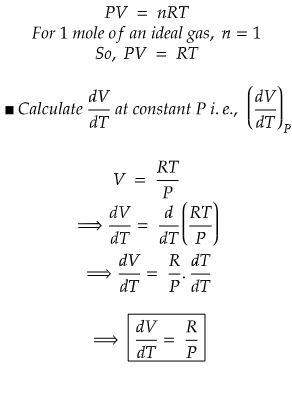
Problem) Show that molar volume, V is an exact differential using the ideal gas equation for 1 mole of gas.
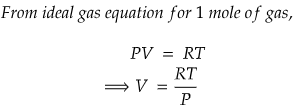
We know that R is universal gas constant, but temperature, T and pressure, P can vary.
So, we can write, molar volume V as a function of P and T.
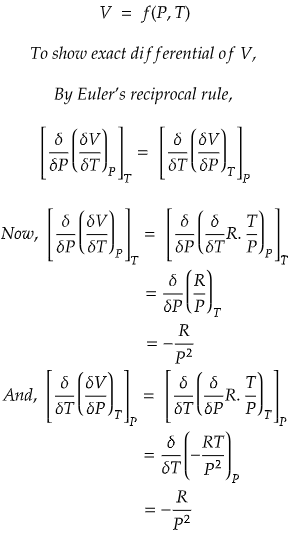
Since, both RHS and LHS are equal, hence we can conclude that molar volume, V is an exact differential.
Van der Waals Gas Equation
Now, let's move onto the Van der Waals gas equation. The Van der Waals equation is a modification of the ideal gas equation that takes into account the finite size of gas molecules and the attractive forces between them. The Van der Waals equation is given by:
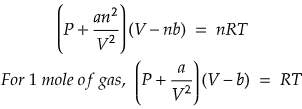
- P is the pressure,
- V is the volume,
- n is the number of moles,
- R is the ideal gas constant,
- T is the temperature,
- a is the Van der Waals constant related to the attractive forces between molecules, and
- b is the Van der Waals constant related to the volume occupied by one mole of the gas molecules.
Now, let us calculate the partial differential of P with respect to T at constant volume, V.
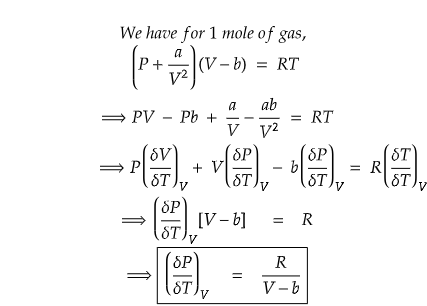
What we learnt?
Understanding exact differentials is significant because they allow for the integration of thermodynamic quantities along a specific path, making it easier to analyze and predict the behavior of the system. In the case of the ideal gas equation, the concept of exact differentials aids in the study of how pressure, volume, and temperature are related in a consistent and well-defined manner for ideal gases.
In contrast to the ideal gas equation, the Van der Waals equation introduces additional complexities due to the correction terms (a and b), making the differentials inexact. This complexity reflects the non-ideal behavior of real gases, where molecular interactions and finite molecular size have a significant impact on the gas properties.
Software used:
The mathematical equations are prepared using mathcha.io editor.

A Deep Dive into Nutrition Essentials: Your Path to a Healthier, Happier You |ChemFam #72|
Decoding Liver Function Tests through Chemistry |ChemFam #71|
Understanding the Dynamic Roles of Metalloenzymes and Metal-Activated Enzymes |ChemFam #70|
Cracking the Thermal Code: Differential Thermal Analysis in Modern Research |ChemFam #69|
Applications and Importance of IR Spectroscopy: Shedding Light on Molecular Structures |ChemFam #68|
The Silent Revolution: How Polymers are Shaping Our World? |ChemFam #67|
Beyond the Bin: The Many Faces of Plastic Management |ChemFam #66|
Spectrophotometry Simplified: The Beer-Lambert Law in Spectrophotometry |ChemFam #65|
Chromatography: Unraveling the Science of Separation |ChemFam #64|
Colorful Clues: The Magical World of Chemical Indicators |ChemFam #63|
Colloids in Action: Impacting Your Daily Life More Than You Think |ChemFam #62|
The Complex Landscape of Opioid Analgesics: Addressing The Concerns |ChemFam #61|
Genetic Engineering: Pioneering Progress or Ethical Predicament? |ChemFam #60|
The Guardians Against Microbial Menace: Antibacterial Agents |ChemFam #59|
The Cholesterol Conundrum: The Story of Statins |ChemFam #58|
Unveiling The Control Of Chemistry: How Hormones Dictate Our Mood |ChemFam #57|
Thermodynamic Versus Kinetic Control of Reactions |ChemFam #56|
Bosons: The Quantum Glue That Holds The Universe Together |ChemFam #55|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery-II |ChemFam #54|
Extraction of Lithium Using Electrode Materials of Lithium Ion Battery |ChemFam #53|
Helium: The First Noble Gas |ChemFam #52|
PS The thumbnail image is being created by me using canva.com


This post has been manually curated by @bhattg from Indiaunited community. Join us on our Discord Server.
Do you know that you can earn a passive income by delegating to @indiaunited. We share more than 100 % of the curation rewards with the delegators in the form of IUC tokens. HP delegators and IUC token holders also get upto 20% additional vote weight.
Here are some handy links for delegations: 100HP, 250HP, 500HP, 1000HP.
100% of the rewards from this comment goes to the curator for their manual curation efforts. Please encourage the curator @bhattg by upvoting this comment and support the community by voting the posts made by @indiaunited..
This post received an extra 2.12% vote for delegating HP / holding IUC tokens.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.