The Science contained in mixing water and edible oil


Water has not accompanied since the formation of the earth, thanks to its chemical and physical composition originated life, today water is a very essential resource for our lives both for consumption and for another wide range of use For life, the fascinating thing about water is that, dear reader, it is characterized by being an inorganic chemical compound made up of two hydrogen atoms (H) and one oxygen (O), what I did not know as a researcher is that water at the Physics is a weak electrical conductor, as well as reader friend is to be a universal solvent and a polar solvent at this point what is unique about this case, a reference base is already made to the parameter that measures its polarity and gives it properties of solubilization of different solutes. On the other hand, we have that water has a behavior of solvation force through hydrogen bonds, it also has a physical behavior level of atomic electrical charge, since hydrogen has a positive bond charge (+) and the molecule of negative oxygen (-), also considering the separation of electrical charges in the same molecule, another particularity of water at the level of chemical properties, Acidity 15.74 pKa, Solubility in water 100%, Dipole moment 1.85 D, me surprised all this important data about how simple the water is observed.

Edible oil is another very essential resource in the home, also widely used in the kitchen, but what do we really know about it, well to my knowledge it can be vegetable, animal and other compound nuts, such as coconut, walnut, olives, fats and carbohydrates, continuing in this order of ideas we have to say the oils in this case edible, they are liquid and viscous, I really did not know their composition, well it is just that this type of oil originates from being a triglycerides, which are esters of a glycerin molecule with three fatty acids, in my case what we use in our house is soybean oil for its pleasant taste, it has abundant polyunsaturated fatty acids, well that is because it has a particularity Chemistry this have more than one double bond between their carbons (Poly-Unsaturated Fatty Acids).
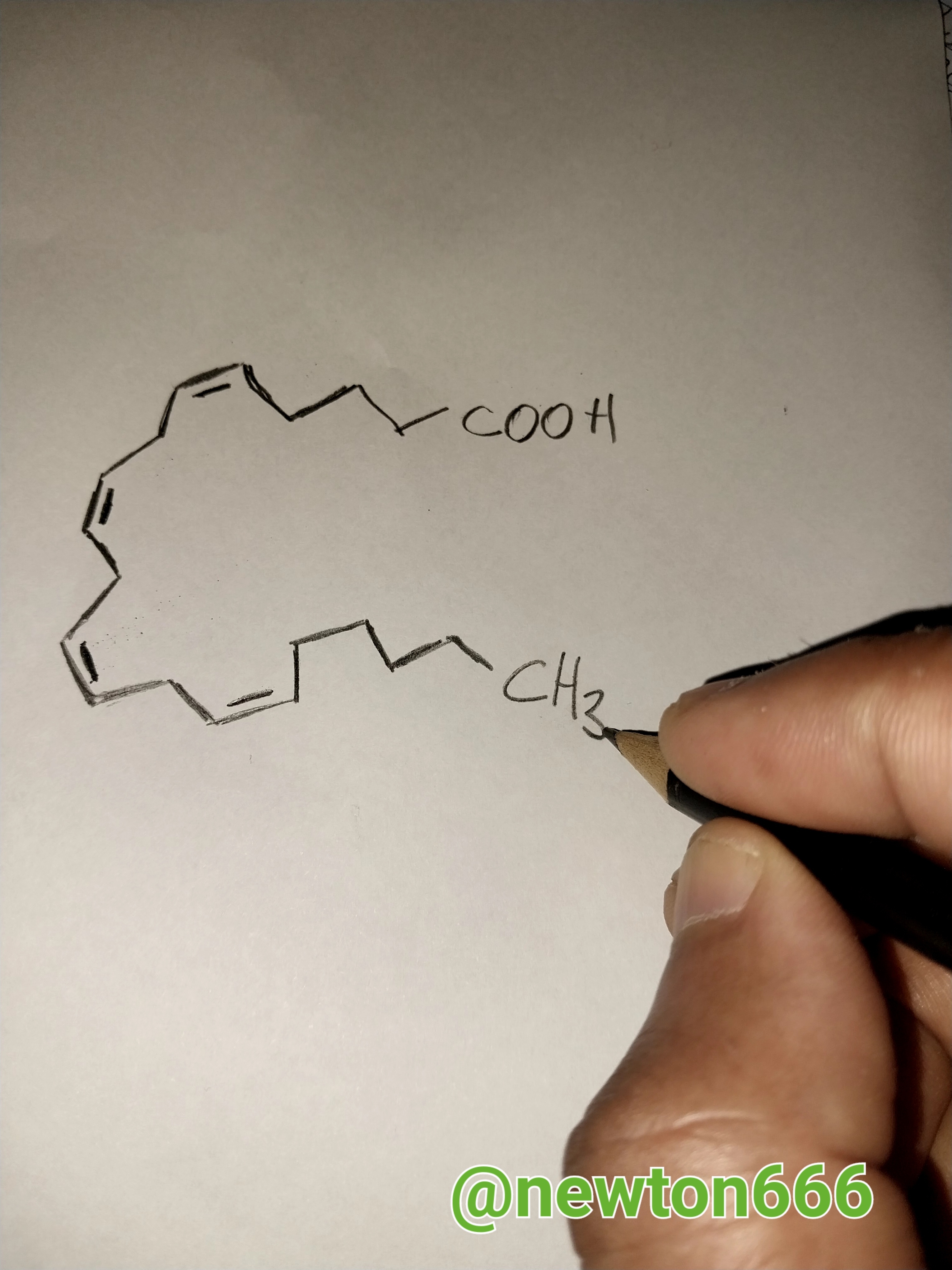
this is a representation to give an example of arachidonic acid, as part of a fatty acid of the omega-6 series with four double bonds. We also have oleic acid.
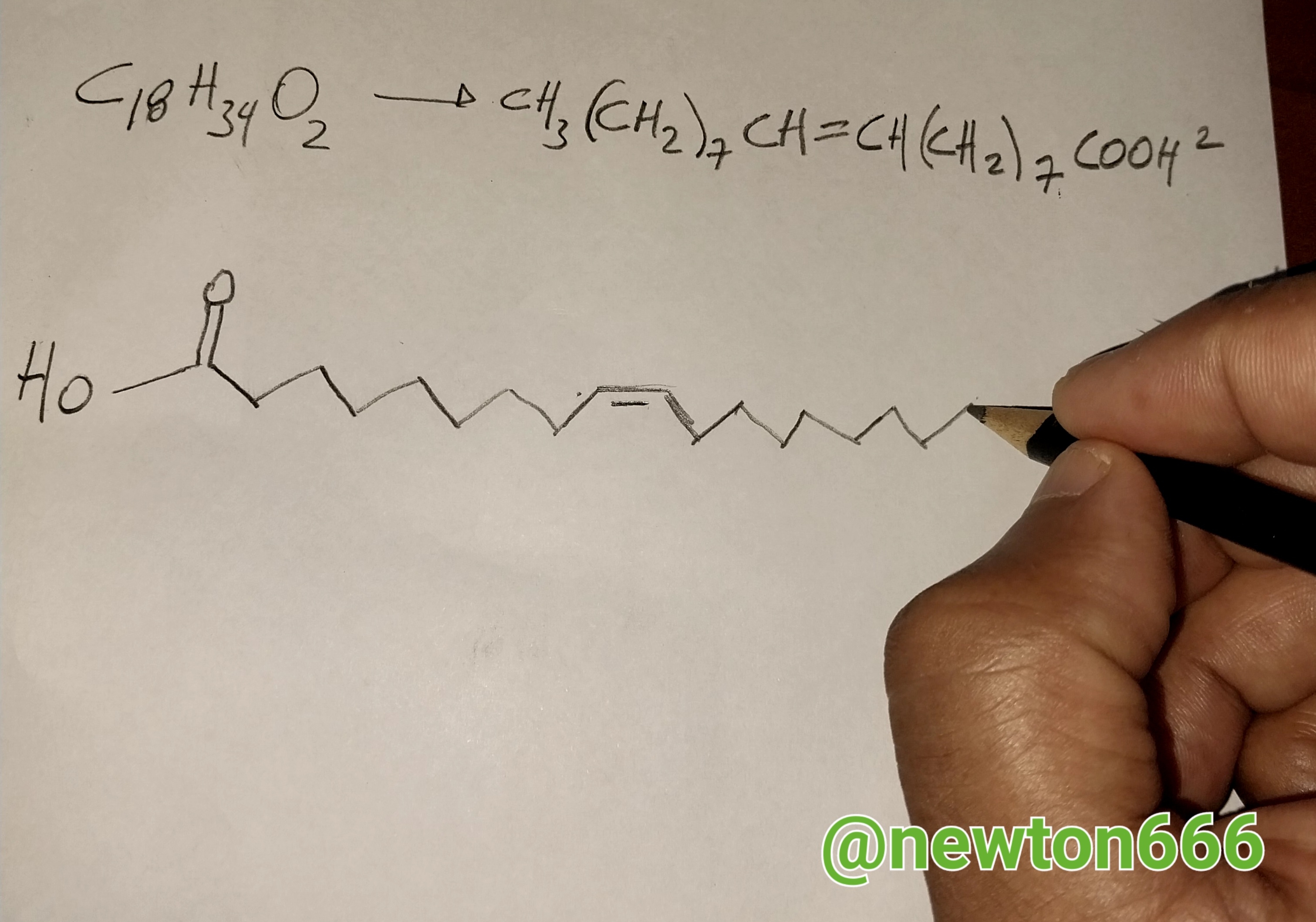
It is good to know as an important fact, in the case of olive, sunflower and soybean oils, this falls under the category of Linoleic acids: CH3-(CH2)4-CH=CH-CH2-CH= CH-(CH2)7-COOH, taking into account or that we cannot forget the information about, which is what makes domestic oils of this type unique are their physical properties in reference to Density 0.9 kg/m³ ; 0.0009 g/cm³, Molar mass 280.24 g/mo, what I like is all the information that a simple soybean oil has, which to fry food, to put it this way, has a lot of content at the science level . Besides, in the same order of ideas, my friend, it is important to know that the greater the number of double bonds, the more unstable the oil is when it comes into contact with the oxygen in the air.

because the water and oil of eating cannot mix, because a physical and chemical phenomenon occurs, when these two substances join, they have different densities, since what it represents as its mass per unit of volume, in the main place also occurs the following reaction is apolar oil does not have charges just as water is a polar compound considering its hydrogen bonds with (+) charge. Now I understand why oil and water cannot be mixed in a simple way, but beware, you have to have knowledge to achieve union by adding an emulsifier, and then separating it by decanting method, which can be used funnel mechanism and a filter.
Bibliographical References
Organic Chemistry Volume 1: Structure and Reactivity
By Seyhan Ege.

A simple way to explain different densities! An important lesson
!1UP
<a href="https://discord.gg/zQrvxAu7mu"> <img src="https://files.peakd.com/file/peakd-hive/thecuriousfool/23wCNFDyCLJu1v77TTg2MYKkd7XWkgF9fhiLujTDAaLaUz7H4AaQkDentB5UMVS8FcrVs.png"></a>If, as I mentioned, why water and eating oil cannot be mixed, because a physical and chemical phenomenon occurs, when these two substances come together they have different densities, since what is represented as their mass per unit volume also has What to see how the hydrogen and oxygen bonds act due to their charge to come into contact with the oil, since the edible oil has acid.
You have received a 1UP from @gwajnberg!
@ctp-curator, @stem-curator, @vyb-curator, @pob-curator
And they will bring !PIZZA 🍕.
Learn more about our delegation service to earn daily rewards. Join the Cartel on Discord.
PIZZA Holders sent $PIZZA tips in this post's comments:
@curation-cartel(5/20) tipped @newton666 (x1)
You can now send $PIZZA tips in Discord via tip.cc!
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.