Sugar's Reaction and Energy Potential
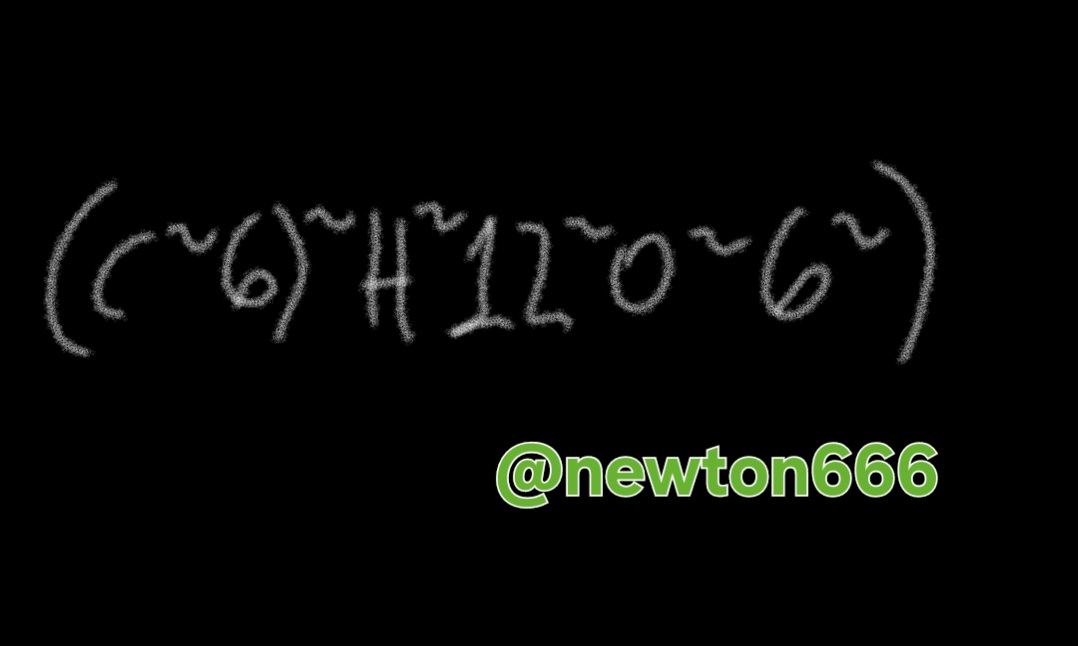
Glucose is important for cellular synthesis, from my fellow readers, shows what to look out for during glycolysis, the process by which cells break down glucose to allow energy to be released. During glycolysis, glucose is oxidized and converted into pyruvate molecules. These molecules can enter the citric acid cycle and produce: ATP (adenosine triphosphate), the main source of energy used by cells
Potassium permanganate Sugar explosion
Potassium permanganate (KMnO ~ 4 ~) Oxygen. When combined with sugars (reducing agents) in the original medium, various oxidative reactions can occur. The reaction that occurs is:
MnO~4~ + e → MnO~4~ (color changes from violet to green)
MnO~4~ + 2 H~2~O + 2e → MnO ~2 ~ + 4 OH ~ + 2 H~2~O + 2e →
MnO ~2 ~ + 4 OH~ (yellow-orange color)
Readers are warned, special attention: Take protective measures when performing this test, wear gloves and glasses.
Sodium Performate (Parchment):
Sodium Performato is a heat-resistant greased paper used for cooking and baking.
It has no special chemical properties, but due to its special properties, its use in cooking can affect the texture and flavor of food.
Ultimately, sugar is an important source of energy for our cells and the chemical reactions vary depending on the conditions and foods involved. While not explosive, mint is excellent for baking and cooking. If you want to delve deeper into a particular topic, don't hesitate to ask.
At the center of this research is chemistry, the study of changes in matter. The reaction between sugar and leather provides an interesting picture of the relationship between organic and inorganic compounds and the power they possess.
The most common form of sugar is sucrose, a disaccharide composed of glucose and fructose. Leather is made primarily from collagen, a fibrous protein found in animal tissues such as skin. When sugar comes into contact with the skin, it becomes very hot, causing what is called caramelization.
Caramelization is a complex process that involves the breakdown of sugars, resulting in the formation of aromatic compounds and dark pigments. During this process, sucrose is broken down into glucose and fructose, which affects the amino acids in the skin's collagen.
This produces many compounds, including caramel compounds, that give color and flavor to caramelized foods.
But is it about power? Caramelization of crust sugars is not only a cooking process, but also affects the release of energy. While caramelization is not an energy source in some sense, it is a decomposition process, meaning it releases energy in the form of heat.
This thermal energy can be used for different purposes. For example, when cooking, caramelization does not change the taste or appearance of food, but it generates heat to help it cook. Furthermore, in a general sense, the caramelization of sugars in the skin can be considered a potential use in biomass production or in industries that require heat.
However, it is important to note that although the caramelization of sugar in parchment can be a source of energy in terms of heat, its efficiency and viability as a renewable energy source are issues that require further research and development. Furthermore, the sustainability and environmental impact associated with sugar production and processing must also be considered in any discussion of its energy potential.
The reaction between sugar and parchment offers a fascinating insight into food chemistry and the energy potential contained in these seemingly disparate elements. From the sweetness of sugar to the resilience of parchment, this interaction reminds us of the complexity and diversity of the world we inhabit, as well as the infinite possibilities that arise when these worlds converge.
Bibliographic reference
Chemistry: the Central Science by Theodore L. Brown, H. Eugene LeMay, Bruce E. Bursten, 2004.
Physics for Science and Technology, Vol. 1C: Thermodynamics by Paul Allen Tipler, Gene Mosca, 2021.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.