Acid rain,causes and effects.
Acid rain is a downpour or some other type of precipitation that is uncommonly acidic, implying that it has raised degrees of hydrogen particles (low pH). It can effectsly affect plants, oceanic creatures, and framework.Acid rain is brought about by emanations of sulfur dioxide and nitrogen oxide, which respond with the water particles in the air to create acids.


A few governments have put forth attempts since the 1970s to diminish the arrival of sulfur dioxide and nitrogen oxide into the climate with positive outcomes. Nitrogen oxides can likewise be created normally by lightning strikes, and sulfur dioxide is delivered by volcanic eruptions.Acid rain has been appeared to impactsly affect backwoods, freshwaters, and soils, slaughtering creepy crawly and amphibian living things, making paint strip, erosion of steel structures, for example, extensions, and enduring of stone structures and sculptures just as having impacts on human health.

Definition
"Acid rain" is a well known term alluding to the affidavit of a combination from wet (rain,snow, mist, cloud-water, and dew) and dry (acidifying particles and gases) acidic segments. Refined water, when carbon dioxide is taken out, has an unbiased pH of 7. Fluids with a pH under 7 are acidic, and those with a pH more prominent than 7 are basic. "Clean" or unpolluted rain has an acidic pH, yet typically no lower than 5.7, on the grounds that carbon dioxide and water noticeable all around respond together to shape carbonic acid, a feeble corrosive as per the accompanying response:
H2O (l) + CO2 (g) ⇌ H2CO3 (aq)
Carbonic acid at that point can ionize in water framing low centralization of carbonate and hydronium particles:
H2O (l) + H2CO3 (aq) ⇌ HCO3− (aq) + H3O+ (aq)
The issue of Acid rain has expanded with populace and industrial development, yet has gotten more inescapable. The utilization of tall smokestacks to decrease neighborhood contamination has added to the spread of acid rain by delivering gases into provincial barometrically circulation. Often affidavit happens an extensive separation downwind of the discharges, with rugged locales having a tendency to get the best statement.A case of this impact is the low pH of downpour which falls in Scandinavia.
Acid rain sources
Natural sources:

The key characteristic natural phenomena that contribute acid creating gases to the air are emanations from volcanoes.
Acid-producing gasses are also created by biological processes that occur on the land, in wetlands, and in the oceans. The major biological source of sulfur compounds is dimethyl sulfide.
Human sources:

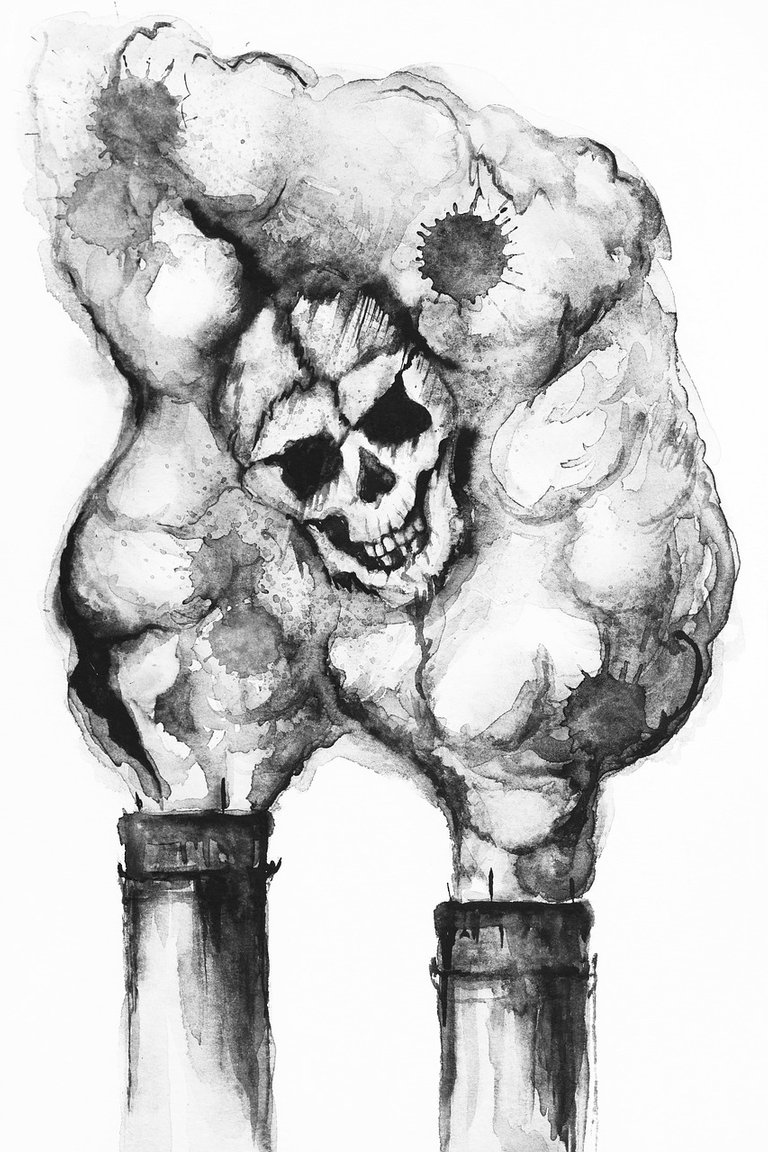
The major reason for acid rain is sulfur and nitrogen mixes from human sources, for example, power age, creature horticulture, production lines, and engine vehicles. Electrical force age utilizing coal is among the best supporters of vaporous contamination answerable for acidic downpour. The gases can be conveyed many kilometers in the climate before they are changed over to acids and saved.

Previously, plants had short channels to let out smoke, yet this caused numerous issues locally; along these lines, industrial facilities currently have taller smoke pipes. Be that as it may, dispersal from these taller stacks makes toxins be conveyed farther, causing inescapable natural harm.
Chemical sources:
In the gas stage sulfur dioxide is oxidized by response with the hydroxyl extremist through an intermolecular reaction:
SO2 + OH· → HOSO2·
which is trailed by:
HOSO2· + O2 → HO2· + SO3
Within the proximity of water, sulfur trioxide (SO3) is changed over quickly to sulfuric corrosive:
SO3 (g) + H2O (l) → H2SO4 (aq)
Adverse effects
Both the lower pH and higher aluminum focuses in surface water that happen because of acid rain can make harm fish and other oceanic creatures.

Acid rain has dispensed with bug life and some fish species, remembering the creek trout for certain lakes, streams, and brooks in geologically touchy regions.
The United States Environmental Protection Agency's (EPA) site states: "Of the lakes and streams over-viewed, acid rain caused corrosiveness in 75% of the acidic lakes and about half of the acidic streams".
Soil science and science can be genuinely harmed by acid rain. A few organisms can't endure changes to low pH and are killed.The catalysts of these microorganisms are denatured by the corrosive. The hydronium particles of corrosive downpour additionally activate poisons, for example, aluminum, and filter away fundamental supplements and minerals, for example, magnesium.

Unfavorable impacts might be in a roundabout way identified with acid rain, similar to the acid's consequences for soil or high grouping of vaporous forerunners to acid rain. High elevation timberlands are particularly defenseless as they are frequently encircled by mists and mist which are more acidic than rain.
Different plants can likewise be harmed by acid rain, however the impact on food crops is limited by the use of lime and manures to supplant lost supplements. In developed territories, limestone may likewise be added to build the capacity of the dirt to keep the pH stable, yet this strategy is to a great extent unusable on account of wild terrains. At the point when calcium is filtered from the needles of red tidy, these trees become less cool open minded and display winter injury and even demise.

Acid rain can make the sea's pH fall, known as sea fermentation, making it more hard for various beach front species to make their exoskeletons that they have to endure. These seaside species interface together as a feature of the sea's natural pecking order, and without them being a hotspot for other marine life to benefit from, more marine life will kick the bucket.
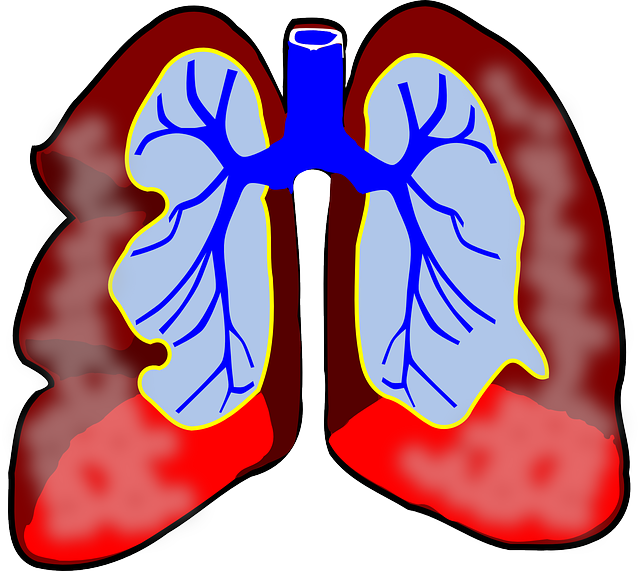
Acid rain doesn't legitimately influence human wellbeing. The acidity in the water is too weaken to even think about having direct unfavorable impacts. The particulates liable for acid rain (sulfur dioxide and nitrogen oxides) do have an antagonistic impact. Expanded measures of fine particulate issue noticeable all around add to heart and lung issues, including asthma and bronchitis.
Ways to limitation of acid rain
Technical solution
Many coal-terminating power stations use flue gas desulfurization (FGD) to eliminate sulfur-containing gases from their stack gases. For a commonplace coal-terminated force station, FGD will eliminate 95% or a greater amount of the SO2 in the flue gases. A case of FGD is the wet scrubber which is usually utilized. A wet scrubber is fundamentally a response tower furnished with a fan that separates hot smoke stack gases from a force plant into the pinnacle. Lime or limestone in slurry structure is additionally infused into the pinnacle to blend in with the stack gases and consolidate with the sulfur dioxide present. The calcium carbonate of the limestone produces pH-unbiased calcium sulfate that is genuinely eliminated from the scrubber. That is, the scrubber transforms sulfur contamination into modern sulfates.
In certain zones the sulfates are offered to substance organizations as gypsum when the virtue of calcium sulfate is high. In others, they are set in landfill. The impacts of acid rain can keep going for ages, as the impacts of pH level change can animate the kept filtering of unfortunate synthetics into in any case flawless water sources, murdering off weak bug and fish species and impeding endeavors to reestablish local life.
All images are free and taken from pixabay.
Thanks for reading,
stay safe,stay healthy.
Hello @
It's one thing to be able to use a resource to guide you in some way. Although I have certainly learned that it is best to write about what you know so that the words are yours.
This is not even paraphrasing, you just change a few words and that's it. Here there is practically nothing that is of your creation, but copies of paragraphs from Wikipedia, and you change a few words. That's not what it's about.
Besides, you're not quoting from phrases you have in the text. It's NOT optional, it's mandatory, but it's simply considered plagiarism.
I will leave you a publication in which you can see how to quote the sources.
I must silence this publication. Because it is not of your creation.
As you can see below, it is practically the same as what is written. It's not about that.