CATALYTIC DISINTEGRATION IN FUELS UPGRADING
(Edited)
At the level of organic chemistry there are more than 18 million known organic compounds, which have their own physical and chemical properties, referring specifically to the melting point, boiling point and chemical reactivity.
So that organic chemistry as a branch of chemistry, is responsible for the study of carbon and chemical reactions associated with this element, knowing that all organic and vegetable matter is constituted by it, hence it has been possible to classify organic compounds according to families based on the properties and functional group present, clearly assuming the structural characteristics of the same.
Image courtesy of: jdn2001cn0
Consequently, this classification is a product of the similarities in the chemical behavior between compounds, which makes it much easier to understand organic compounds, since we avoid the fact of handling isolated compounds whose properties could be random, so it is understandable that organic compounds appear in families since they share affinities that can be studied together.
Consequently, those characteristics of structural origin that make it possible to classify organic compounds, have been called functional groups, which respond to a set of atoms or molecules that have specific chemical behaviors, in other words, a functional group behaves similarly in the different molecules where it is present, therefore, at the level of organic chemistry we can get simple functional group as in the case of double and triple bonds, or much more complex groups such as the carbonyl group of acids, ketones or aldehydes.
In this sense, it must be taken into account that the chemical functioning of an organic molecule, regardless of its size, length or complexity, is determined by the functional group present in the molecule. A clear example of this can be predicted through the functioning of the compound ethylene, understood as that chemical compound whose functional group is a double bond, capable of participating in reactions very similar to that of cholesterol, which is a much more complex molecule, but which also has a double bond in its structure, so that both molecules react in the same way in a halogenation process.
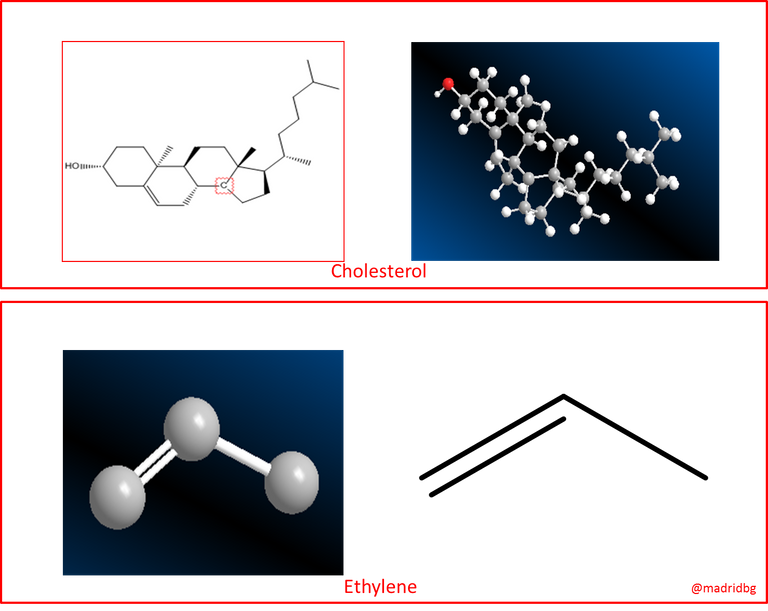
Fig. 2: Cholesterol and ethylene molecule.
In this sense, we will focus our attention on the study of those compounds related to alkenes, alkynes and specifically alkanes, since through these we can obtain the mixture of hydrocarbons necessary to provide the respective octane rating to the fuels we use today.
Consequently, we must be aware that alkanes are characterized by presenting in their structure only single bonds, while alkenes double bonds and alkynes can generate the formation of triple bonds.
These compounds as a whole, have great affinities according to the properties that provide their structures, so that those where double and triple bonds appear we say that they are unsaturated hydrocarbons and in their absence the alkanes that are constituted by single bonds, are hydrocarbons of saturated type also known as aliphatic since through the Greek the word is associated with fats.
In this sense, and according to the International Union of Pure and Applied Chemistry, all organic compounds including hydrocarbons, present a series of nomenclatures which we must follow to establish a specific name for these compounds and although the objective of this writing is not to focus on the nomenclature, we will make an opening where we will explain very superficially what this process consists of and which we will develop in a series of steps so that it is much more pleasant and flexible for the reader.
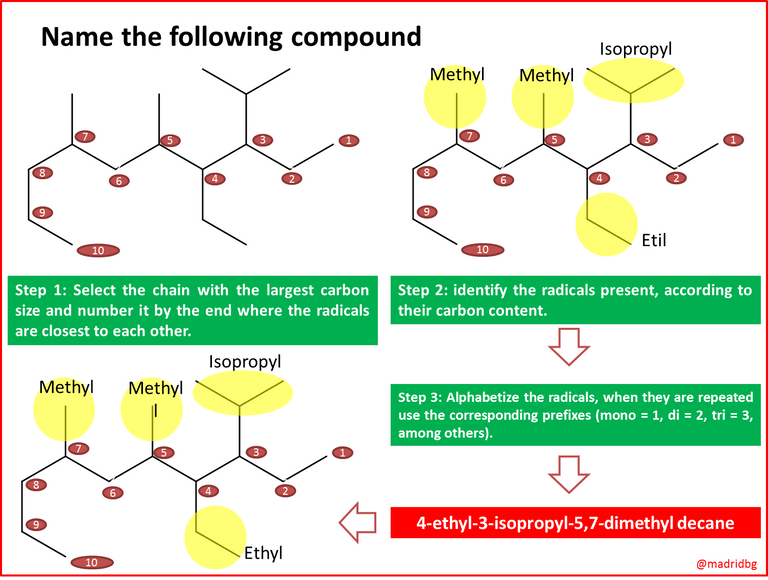
Fig. 3: Cholesterol molecule and ether.
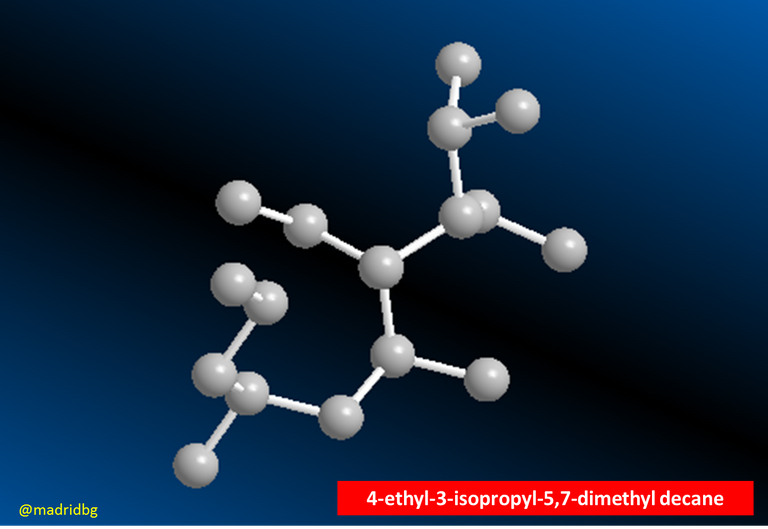
Fig. 4. 4-ethyl-3-isopropyl-5,7-dimethyl-decane.
Therefore, it is necessary to understand that alkanes are also known as kerosenes because of the strong affinity that these compounds present, although they usually produce specific reactions with oxygen, which are of utmost importance in the combustion process and which have a direct relationship with regard to the use of fuels, since they generate sufficiently high enthalpies, as well, both the boiling point and melting point increase in relation to the amount of carbon atoms present, so we can establish that the melting point and boiling point is directly proportional to the amount of carbon atoms, so that those molecules that exhibit large amounts of carbon are expected to have a high melting point and boiling point.
So, referring to the combustion capacity of hydrocarbons, we must remember that we have managed to extract these compounds from oil, providing the possibility of producing useful derivatives such as gasoline, which provides great development alternatives to mankind, hence natural gas and oil represent the largest natural source of hydrocarbons, specifically alkanes. Likewise, it is necessary to take into account that these sources or storage of these organic compounds are products of the decomposition of organic and vegetable matter that has been taking place millions of years ago.
Consequently, we must know that natural gas is assumed as a mixture formed mainly by methane, although we can also get propane and butane in smaller proportion, for its part, oil is presented as a much more complex mixture of hydrocarbons, which in the first instance is separated through distillation processes in smaller fractions that we can use for the benefit of humanity.
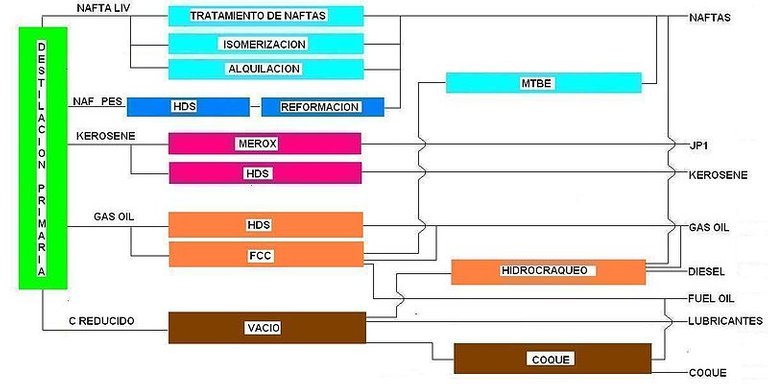
Diagram of a refinery: Desost
The refining process of the latter begins through the distillation of oil, being carried out in three main fractions, firstly a direct gasoline whose boiling point varies from 30 to 200 °C, kerosene whose boiling point is from 175 to 300 °C and finally, diesel oil with points from 275 to 400 °C, further on, we can execute controlled distillation processes at reduced pressures with the purpose of obtaining lubricants, waxes and oils that end up generating asphalt as a residue.
In this long industrial system, it is necessary to understand that the distillation of oil is only the first step in the production of gasoline, since even though in the first instance direct gasoline is obtained, it is as fuel does not work properly because it causes a knocking in the engine, which originates as a consequence of the uncontrolled combustion of the bad fuel, a process that can be initiated due to a hot surface of the cylinder before the spark that emits the spark plug is produced.
So this pre-ignition is what manifests itself as a knocking in the engine that can destroy it as a result of the irregular forces on the crankshaft and the high temperatures that are acquired in the engine.
Consequently, and to avoid this problem that affects vehicles so much, a process has been established to measure the octane number of fuels, which represents a measure of the antiknock properties of the same, which is why, despite being a low molecular weight hydrocarbon, heptane does not work properly as fuel since its octane number is zero, which means that it is bad as fuel, while 2,2,4-trimethylpentane is assigned an octane number of 100 for its good antiknock properties.
Hence, direct gasoline is starting to be left aside due to its anti-knock properties and now the use of kerosene is being evaluated, which has to undergo a catalytic disintegration process, which means, in short, taking fractions of it, which have a low boiling point, breaking them into smaller molecules and recombining them with existing alkanes, thus providing greater usefulness and efficiency in fuels.
So this energetic process is providing greater efficiency and durability to the engines used today and all thanks to scientific advances in this area.
BIBLIOGRAPHY CONSULTED
[1] Chang, R. (2010). Química. Decima edicion. McGraw-hill Interamericana editores. ISBN: 978-607-15-0307-7.
[2] Ralph, H. Petrucci, William S. Harwood, E. Geoffrey Herring. (2003). QUIMICA GENERAL. Octava edición. PEARSON EDUCACIÓN. S.A., Madrid.
[3] WADE,LEROY. (2011). . ORGANIC CHEMISTRY. VOLUME 2. SEVENTH EDITION. PEARSON EDUCATION, MEXICO, 2011 ISBN: 978-607-32.()793-5.
OF INTEREST
For more information related to the areas of science, technology, engineering and mathematics, do not hesitate to visit #stemsocial and #stem-espanol, they are communities that promote scientific advances in these areas

0
0
0.000
https://twitter.com/279290402/status/1613954252724133894
The rewards earned on this comment will go directly to the people( @madridbg ) sharing the post on Twitter as long as they are registered with @poshtoken. Sign up at https://hiveposh.com.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.