THE CHEMICALS OF LIFE: Back in time to ‘RNA world’
All new organisms come from other organisms –
they don’t just arise spontaneously. So how did life first come about? Could it
have developed from non-living chemicals? Some experiments, carried out in the USA
in the 1950s by Stanley Miller, showed that this is a definite possibility. In
his laboratory, Miller recreated the conditions thought to have been present on
Earth 3 500 million years ago. Then, the atmosphere contained no oxygen. Instead,
it was probably a mixture of methane, ammonia, carbon dioxide, hydrogen sulfide
and hydrogen. There were frequent electrical storms, and no ozone layer to
protect the surface of the Earth from the Sun’s ultraviolet rays.
When these conditions were recreated in the laboratory, the chemical reactions in the mixture produced several biological molecules: sugars, amino acids and bases. The findings were important because these molecules are the building blocks of nucleic acids and proteins – the chemicals on which all life on Earth is based. Since these groundbreaking experiments, further work over the past half century has suggested that one of the earliest self-replicating ‘life-forms’ may have been RNA. An ‘RNA world’ may have persisted for a long time, until conditions became milder and DNA was able to take over. However, we still have many jigsaw pieces to find before we work out how these complex molecules might have made the giant step to become life.
STUDYING THE CHEMICALS OF LIFE
The study of the chemicals and reactions that take place inside living organisms is a science in its own right- biochemistry. Biochemists aim to understand how living organisms work by studying how the molecules within their cells interact. In this post, I will look at carbohydrates, lipids, proteins and nucleic acids and describe the characteristics that make them the chemicals of life.
SOME CENTRAL THEMES IN BIOCHEMISTRY
The chemical systems that make up living things seem incredibly complicated. but there are some simple underlying patterns:
- Living organisms contain a huge range of macromolecules, but these large molecules are built from a small number of simple molecules.
- The simple building-block molecules are very similar in all organisms, suggesting that all life had a common origin.
- The characteristics of an organism are determined by the information contained in its DNA.
- The DNA contains information that the cell can use to make proteins. Many proteins are enzymes, which control the physical and chemical activities of an organism.
- The chemical activities that go on inside an organism are given the general term metabolism.
- Metabolic reactions are divided into two general categories: anabolic and catabolic. Anabolic reactions build up large molecules from smaller ones while catabolic reactions do the reverse, breaking down larger molecules.
- Anabolic reactions usually involve condensation reactions in which building-block molecules are joined together and a water molecule is released.
- Catabolic reactions, such as those that occur during digestion, usually involve hydrolysis reactions in which larger molecules are split as they react with water. In photosynthesis, plants use the energy from sunlight to build up organic molecules such as sugars from simple ones such as carbon dioxide and water.
- All organisms need a supply of energy, which they obtain via respiration. In respiration, organic molecules are oxidised into simpler molecules, usually carbon dioxide and water. The resulting energy is used to fuel the many energy-requiring processes within the organism.
Let’s not forget that water is very essential to humans, as it is to all living organisms. Life evolved in water, most biochemical reactions take place in solution and all organisms that live on land – like ourselves – can do so because they have their own aquatic environment inside them.
All of the living cells in the human body contain water and are surrounded by it; in fact, most of the human body is made up of water. Even the most complex organ – the human brain – is about 85 per cent water.
LIFE ON EARTH IS CARBON-BASED
Why is life on Earth based on carbon? It is because this element can bond with itself repeatedly, to produce an infinite variety of molecules. Living things are composed mainly of organic molecules: proteins, carbohydrates, lipids and other molecules that have ‘skeletons’ made from carbon.
The carbon atom
Carbon has an atomic number of 6: it has a nucleus containing six protons and six neutrons, orbited by six electrons, two in an inner shell, the other four in an outer shell. Carbon acquires a full, and therefore stable, outer shell of eight electrons by sharing four more. So, carbon forms four covalent bonds – bonds with electrons shared between the atoms. We say it has a valency of 4.
Carbon can form covalent bonds with other carbon atoms to form stable chains or rings. C-C bonds are the most common; compounds that contain single carbon bonds are said to be saturated. C=C bonds are also frequent and C≡C are possible. Compounds with double or triple bonds are said to be unsaturated. This means that they are not saturated with hydrogen (more hydrogen atoms could be added at their multiple bonds). This is particularly relevant with respect to the fatty acids, as we see later in this chapter.
Let’s also remember that atoms have electrons in shells. Two electrons fill the first shell; eight electrons fill the second shell. Atoms with a full outer shell are stable. Atoms with a less than full outer shell are reactive and combine with others in order to fill the outer shell.
So, carbon atoms commonly form covalent bonds with hydrogen, nitrogen, oxygen, phosphorus and sulphur. These elements make up the vast majority of organic molecules.
What is an organic molecule?
The word (as in ‘organic’ vegetable) is much used today, but the use of the word to mean ‘no added chemicals’ is not scientific. Originally, the word organic meant ‘of living origin’, in a time when scientists thought that the chemicals that made living things had a divine origin and could not be made by people. When scientists started to make organic molecules in the laboratory (urea was first) this idea was abandoned.
For our purposes, we can say that organic molecules are ones with carbon backbones – at least one carbon bonded to another one. Some organic molecules are enormous, with relative molecular masses in the millions. Some simple examples of organic compounds are ethane, ethanol, ethanoic acid, glucose and amino acids while some examples of simple inorganic compounds are water, nitrogen gas, ammonia, carbon dioxide, hydrogen sulphide and nitrate ion.
In this chapter, we look at four types of large organic molecules commonly found in living things: carbohydrates, lipids, proteins and nucleic acids.
CARBOHYDRATES
Carbohydrates are organic molecules that contain three elements: carbon, hydrogen and oxygen. Carbohydrates include sugars and starches. There are three basic types of carbohydrate molecule named according to structure and size:
They are monosaccharides: single sugars, disaccharides, which are double sugars (made from two monosaccharides), and polysaccharides, which are multiple sugars (polymers of many monosaccharides). A monomer is a single molecule.

A dimer is formed when two of the same molecules link together, while a polymer is formed when many molecules link together. Carbohydrates are the first molecules made in photosynthesis. Lipids, Proteins and nucleic acids are formed from carbohydrates. And the term ‘carbohydrate’ covers a range of chemicals which includes sugars, starches and cellulose. The picture beside shows foods containing complex carbohydrates – these foods are staples of the human diet.
MONOSACCHARIDES AND DISACCHARIDES
Monosaccharides and disaccharides are classed as sugars and usually have names ending in -ose, such as sucrose and lactose. In monosaccharides, the three elements carbon, hydrogen and oxygen are always present in the same ratio and they have the basic formula (CH2O)n. Monosaccharides are classified according to the number of carbon atoms they have: three, five and six are the most usual. In glucose, for example, n is 6, so its formula is (CH2O)6 or C6H12O6.
A table showing some common carbohydrates
| CHO | Compound | Sub-units | Occurence in living things |
| Monosaccharide | glucose | widespread | |
| fructose | sweet fruits | ||
| galactose | milk | ||
| Disaccharide | maltose | 2 × glucose | germinating seeds |
| sucrose | glucose + fructose | fruits | |
| lactose | glucose + galactose | milk | |
| Polysaccharide | starch | glucose | plants (storage) |
| glycogen | glucose | animal (storage) | |
| cellulose | glucose | plant cell walls |
Examples of common monosaccharides according
to the number of carbon atoms are triose with 3 number of carbon atoms
(e.g glyceraldehyde), pentose having 5 carbon atoms (e.g ribose, deoxyribose
in RNA and DNA) and, hexose with 6 carbons atoms (e.g glucose, fructose,
galactose).
Glucose
It is useful to begin a study of carbohydrates with glucose: it is the main source of energy for many organisms – including humans and most of the common polysaccharides are glucose polymers. Glucose is a hexose (6-carbon) sugar that has the formula C6H12O6. All other hexose sugars, such as fructose and galactose, have the same formula.
CHROMATOGRAPHY
Chromatography is the general name given to a number of techniques that can separate and identify the components of a mixture. Paper chromatography is a technique that separates the components of a mixture according to the solubility in a particular solvent. It is useful when only tiny amounts of each substance are present and may not be detectable by one of the biochemical tests. It can also distinguish between substances that give the same result in biochemical tests. For example, the Benedict’s test gives a positive result for both glucose and fructose because both are reducing sugars, but chromatography can separate them very easily.
Doing chromatography
In a figure of a basic chromatography tank. The paper is like blotting paper and has a pencil line at the bottom – the samples are dotted onto the paper along this line at regular intervals. Each sample is allowed to dry before adding the next, to avoid spreading and merging of the substances under test. When all the samples have been put on the paper, the bottom end is lowered into the solvent at the bottom of the tank. The lid is put on to prevent evaporation of the solvent, and the apparatus is then left for a few hours to allow the solvent to soak up the paper.
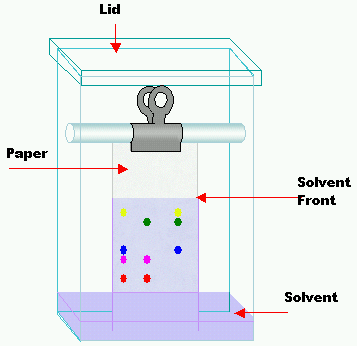
Different substances dissolve in a particular solvent to different degrees – a substance that dissolves very well will move further up the paper, perhaps to near the top. A substance that hardly dissolves at all remains near the original spot placed on the paper. If you are separating out pigments, for example, from a leaf extract, it is easy to see where the different substances are on the paper. However, if the test substance is colourless, you just end up with a piece of white paper – this must be treated with a stain to reveal the spots. For amino acid or protein mixtures, the paper is sprayed with ninhydrin which shows up the spots. This is highly toxic, so spraying is always carried out in a fume cupboard.
Identifying your substance
The paper with separated substance is called a chromatogram. Substances can be identified by working out their Rf value, i.e
Rf = distance moved by spot ÷ distance moved by solvent front
Rf values vary between 0 (didn’t move at all) and 1 (went as far as the solvent front). A particular compound will always have the same Rf value in a particular solvent. So, for instance, every time you run lysine in the solvent propanone, it will give you exactly the same Rf value.
TWO-WAY CHROMATOGRAPHY
Sometimes, two substances will have the same Rf values in a particular solvent, so they won’t be separated out. The problem is overcome by using two-way chromatography. The first chromatogram is produced in the usual way but then the paper or plastic sheet is turned through 90° and run again with a different solvent. This reveals extra spots, not distinguishable in the first chromatogram.
BIOCHEMICAL TESTS
Foods are usually mixtures of carbohydrates, lipids and proteins; one way to find out what types of molecule a food contains is to do biochemical tests. Some of the common ones are shown in the table below.
Some simple biochemical tests
| Nutrient | Reagent used | How test is carried out | Positive result |
| Reducing sugar | Benedict’s solution | Add Benedict’s solution to sample in a test tube. Heat in a water-bath | orange-red precipitate |
| Non-reducing sugar | Hydrochloric acid and Benedict’s solution | Once the reducing sugar test has proved negative, boil with dilute acid. Add sodium hydrogencarbonate to neutralise. Carry out reducing sugar test, as described above. | Orange-red precipitate |
| Starch | Iodine solution | Add a few drops of iodine solution to the sample. | Blue-black staining |
| Lipid | Ethanol | Shake the sample with ethanol in a test tube. Allow to settle. Pour clear liquid into water in another test tube. | Cloudy-white emulsion |
| Protein | Biuret solution | Add biuret solution to sample in a test tube. Warm very gently | Lilac/mauve colour |
REFERENCES
https://en.wikipedia.org/wiki/RNA_world
https://www.ncbi.nlm.nih.gov/books/NBK26876/
https://study.com/academy/topic/characteristics-chemicals-of-life.html
https://en.wikipedia.org/wiki/Biochemistry
https://science.jrank.org/pages/859/Biochemistry.html
https://en.wikipedia.org/wiki/Carbon-based_life
https://www.nbcnews.com/mach/science/silicon-based-life-may-be-more-just-science-fiction-n748266
https://en.wikipedia.org/wiki/Carbon
https://www.sciencedirect.com/topics/chemistry/carbon-atom
https://www.dictionary.com/browse/organic-molecule
https://en.wikipedia.org/wiki/Organic_compound
https://www.britannica.com/science/carbohydrate
https://www.livescience.com/51976-carbohydrates.html
https://www.ivyroses.com/HumanBiology/Nutrition/Types-of-Sugar.php
https://en.wikipedia.org/wiki/Disaccharide
Hello,
Your post has been manually curated by a @stem.curate curator.
We are dedicated to supporting great content, like yours on the STEMGeeks tribe.
Please join us on discord.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @minnowbooster.
If you appreciate the work we are doing, then consider supporting our witness @stem.witness. Additional witness support to the curie witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having used the steemstem.io app and included @steemstem in the list of beneficiaries of this post. This granted you a stronger support from SteemSTEM.