Coordinate covalent Combination - with an example.

Introduction
I have been discussing about covalent bonding in the last two articles and we have seen various types and example of molecules formed through them. There is one more type of covalent bonding that is left to be discussed and that is the essence of this article. That one is called Coordinate covalent bonding or combination.
In coordinate covalent bonding, there is a sharing or electrons among reacting atoms. However, there is a slight difference in how electrons are shared. In normal covalent bonding, the reacting atoms donate electrons to be shared. But in coordinate covalent bonding, only one of the elements donate their electrons for sharing. When there is such a situation where only one atom of the pair donates electrons to be shared, it is called a lone pair.

Example of coordinate covalent bonding - Oxonium ion
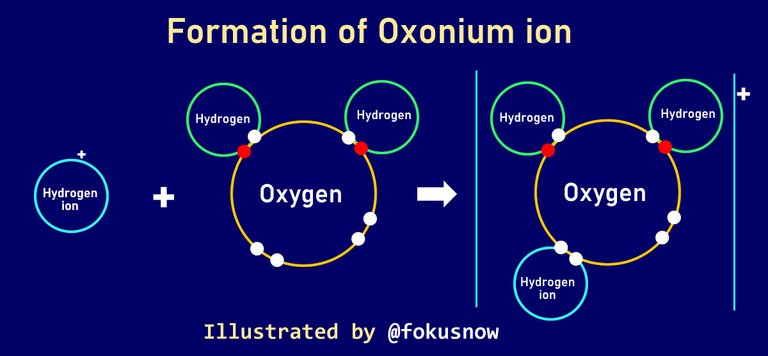
We will use the formation of oxonium ion in water to explain how coordinate covalent bonds operate. In this bonding, 3 hydrogen atoms combine with a single atom of oxygen in such a way that all the atoms attain a stable electronic configuration to for oxonium ions. This is how it works:
The oxygen atom has a lone pair of valence electrons. it means that it will provide those two valence electrons to be shared by the hydrogen atoms. Already since the liquid is water, two of the hydrogens already shares two pairs of electrons with one of the oxygen to form the water. Then, hydrogen ion transfers its positive charge to the third oxygen atom, while the atom shares its two lone pairs with the hydrogen ion.
In essence, there are 4 atoms that combines to form the oxonium ion. 3 hydrogen atoms and one oxygen atom. Two of the hydrogen atoms shares 2 pairs of electrons with the oxygen. The third hydrogen receives a lone pair donation of 2 valence electrons from the oxygen. The oxonium ion receives its positive charge from the positively charged hydrogen ion.
You can consider the diagrammatic representation of the equation below

Formation of Carbon Monoxide (CO)
This is another example of coordinate covalent bonding, although this is not in the absolute sense. Remember that in the formation of carbon monoxide, there are just two atoms involved. One of the atoms is carbon and the other is oxygen.
Remember that these two atoms have to share their valence electrons in order to attain a stable electronic configuration in this combination. Carbon with an atomic number of 6 with 4 valence electrons ready to combine. On the other hand, oxygen has an atomic number of 8 with 6 valence electrons.
There is triple bonds between the carbon and oxygen atoms. The first is a normal covalent bond bond in which the atoms shared two pairs of electrons between each other. The third is a coordinate covalent bond where the oxygen donates its lone pair of electrons to be shared with carbon. In this way, we have 3 bonds holding the two atoms together to for Carbon monoxide. You can understand this more is you look at the diagram below used to represent the bonding.

Properties of Coordinate covalent bonds
Compounds formed from coordinate covalent bonds are very important in many aspects of chemistry. However, there properties are very similar to those of normal covalent bonds. They usually struggle to dissolve in water, but are very readily soluble in non-polar solvents.
Other properties shared with covalent compounds include that they are usually crystalline in shape. Hardly do they conduct electricity. One slight deviation in properties is that they are not as volatile as their covalent counterparts. This is because the strong presence or a coordinate bond in compounds greatly reduces their volatility.

Conclusion
We have seen another type of chemical combination in Coordinate covalent bonds. Only one of the reacting atoms donates the electrons to be shared. An there are many important compounds formed with this type of combination including Carbon monoxide.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.