Chemical bonding - electrovalent combination


Introduction
Taking a good look at the periodic table of elements show that there some elements that have already attained a stable octet or duplet structure. It simply means that they have their outer shells filled out with the complete number of electrons. The rare gases like Neon and Argon are very good examples. Take a look below at their electronic configurations:
| Name of rare gas | Atomic number | Shell distribution |
|---|---|---|
| Neon | 10 | 2,8, |
| Argon | 18 | 2,8,8 |
If we draw the diagrammatic representation of the above two rare gases, you will notice that their inner and outer shells are filled completely with the maximum number of electrons - 8.
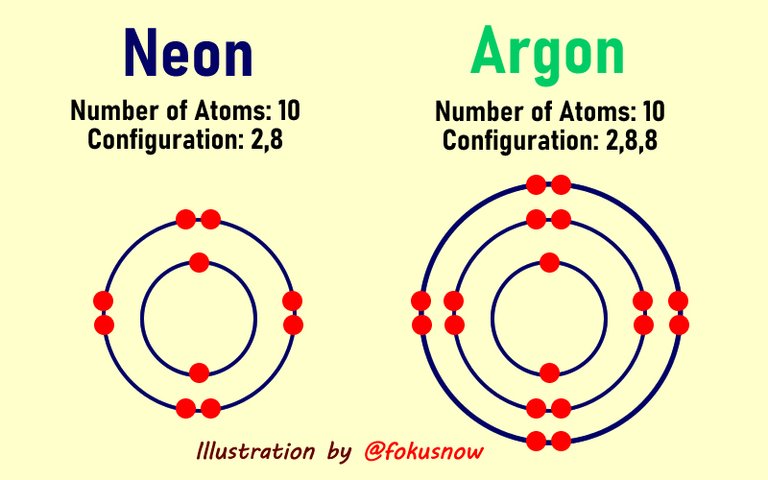
Rare gases and other elements that already has their outer shells filled to the maximum number of 8 are less reactive because they have attained the duplet or octet structure. They do not need to loose or gain any electrons because their shells are complete. However, that is not the case with other elements.
Metals and non-metals whose outermost shells are not complete are more reactive because they are eager to have them filled. This leads to their being very reactive and hence ready to chemically combine with other elements in order to have complete outer most shells. So chemical bond is the combination of elements with other elements in order to have their outermost shells filled and achieve stable electronic configuration.

Electrovalent combination
Electrovalent combination is one of the many types of chemical bonding. Here, there is the transfer of electrons between two elements as they attempt to achieve a stable structure. One of the two elements act as a donor and it is usually a metal. The other atom acts as an acceptor of the donated electron.
The donor achieves a stable configuration by transferring or giving away the excess electron in its outermost shell. While the acceptor also needs that 1 extra electron from the donor to have a stable configuration. At the end of the reaction, both the donor and the acceptor have 8 electrons complete in their outermost electrons.
Donor atoms or elements are observed to be vary reactive and they easily form compounds in any chemical system. This is because it requires very little chemical energy to release the excess 1 electron in their outermost shell. Their valence electron is just one. Examples of elements like this includes metals like potassium and sodium each of which have 1 valence electrons.
Metals that have more valence electrons are usually less reactive because more energy would be needed to dispose the two or more valence electrons they have. Such metals include aluminum with 3 electrons in its outermost shell and calcium with 2.
The situation applies to acceptors of the donated electrons. Usually, non-metals that needs the least number of electrons to attain the octet structure are much more reactive than those that require more. For example, chlorine very readily combines with metals chemically because it just needs 1 valence electron to have a balanced electronic configuration. Florine also has 7 electrons in its outermost shell, requiring just 1 valence electron to be stable. Like chlorine, it is also very reactive.
After a chemical bonding, donors of electrons attain a positive electrical charge. Elements that accept the donated electrons become negatively charged. After a chemical combination involving donors and acceptors, a new compound is formed. These compounds are referred to as electrovalent compounds, owing to the fact that an electrovalent combination was what led to their formation.

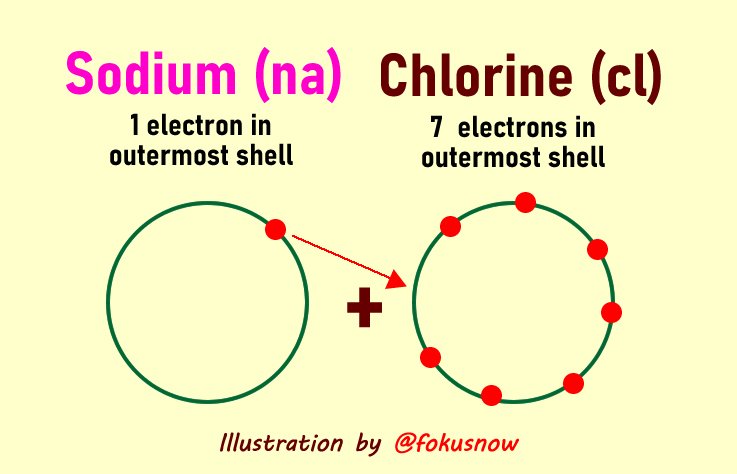
Formation of Sodium Chloride
Sodium chloride is the common salt, an electrovalent compound formed by the combination of sodium and chlorine atoms. Sodium has an atomic number of 11, so it has an extra 1 electron in the outermost shell to donate. Chlorine on the other hand has 7 valence electrons. By accepting 1 electron from sodium, it obtains its octet structure.
The equation
Na => Na+ + e- (As sodium donates an electron, it becomes positively charged.)
Cl + e- => Cl- (As chlorine accepts one electron from sodium, it becomes negatively charged)
Combining the two equations above, we have
Na + Cl - Na+Cl-
You can the diagram of this equations below:
Properties of Electrovalent compounds
Below are some of the observable properties of electrovalent compounds
1. They are usually solids at room temperature
2. They are crystals mostly
3. They are brittle and hard
4. Pressure shatters the crystal structure of electrovalent compounds but the shape is never changed.
5. The forces of attraction in these compounds are strong. Hence, they have very high boiling and melting points. Strong energy is required to break the bonds.
6. They are mostly transparent.

Conclusion
Chemical bonding is very important as it leads to the formation of many new compounds and the attainment of stable electronic configuration by elements. Electrovalent bonding is just one example. There are other types such as covalent bonding which will be discussed in the next article.
Chemical bond is one of the forces that holds atoms together as in formation of molecules. I will like to get to read your subsequent article on bonding and it's properties. This, brought back some forgotten things in Chemistry.
Sure chemistry is sweet and this topic is very important in the formation of certain compounds. I will keep you updated in future articles.
Please do not fail to do that, I do put interest in chemistry.
Congratulations @fokusnow! You have completed the following achievement on the Hive blockchain And have been rewarded with New badge(s)
Your next target is to reach 8750 replies.
You can view your badges on your board and compare yourself to others in the Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPCheck out our last posts:
Lets do it! More is better!
Go @fokusnow, go!
👯💃👯💃👯💃
Thank you for this insightful breakdown of electronic configurations and chemical bonding, particularly in the context of electrovalent compounds. It is no doubt that certain elements with complete outer shells are less reactive due to their stable electronic structures, while others with incomplete outer shells are more eager to bond with other elements to reach stability.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.
Reminds me of when I was so young and in love with chemistry. I and electrovalent bond had a chemistry