Chemical bonding - Covalent combination

Introduction
Yesterday I wrote about electrovalent combination as one of the types of chemical bond in chemistry. We saw examples and illustrations too. If you had missed that article and want to check it out, click here. So today, we will look at another important type of bonding seen in elements - covalent combination.
Remember that in electrovalent combination, there is a transfer of electrons from a donor to the acceptor element. But in covalent combination, there is not transfer of electrons from one element to another. Instead, there is the sharing of electrons between to atoms as they attempt to have a stable octet structure. The formation of some molecules of elements follow covalent combination. Two atoms might share just one electron or a pair of electrons in covalent bonding in order to have a stable configuration.
Each of the reacting elements in a covalent bond contributes the electrons to be shared - either a single electron or a pair. Covalent bonding is the popular combination used by atoms to form molecules of the same element. For example, Two hydrogen atoms must combine in order to form a hydrogen molecule. The same is true of oxygen where the two oxygen atoms combine and contribute their electrons to form the oxygen molecule.

Types of Covalent combination
There are basically three types of covalent combination or bonding. They are as follows:
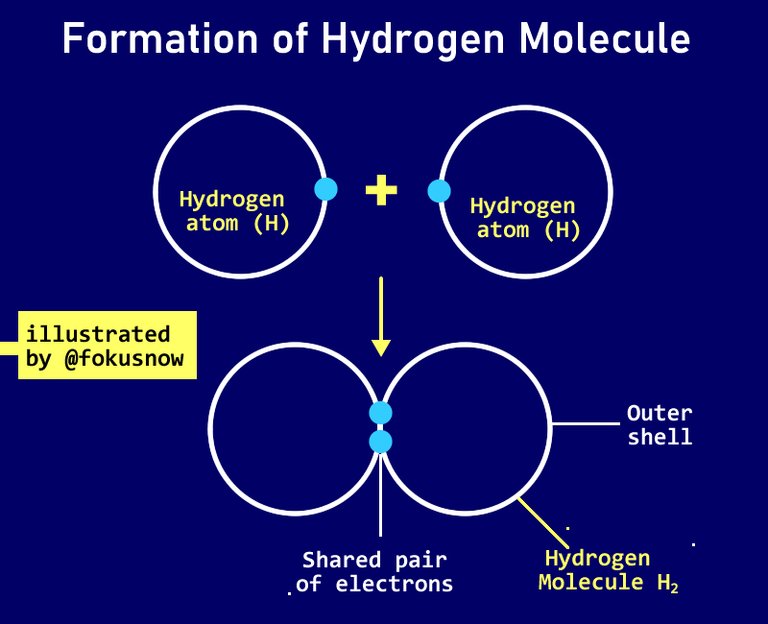
- Single covalent bonds: This type of covalent bonding involves two atoms contributing just 1 electron each in the reaction. When this happens, it is referred as single bond or single covalent bond. There are many examples of this. The most prominent is in the formation hydrogen molecules.
To form hydrogen molecules H2, two hydrogen atoms combine. Each donates one valence electron and the two shares the donated electron to for a hydrogen molecule. Check the diagrammatic representation of this below:
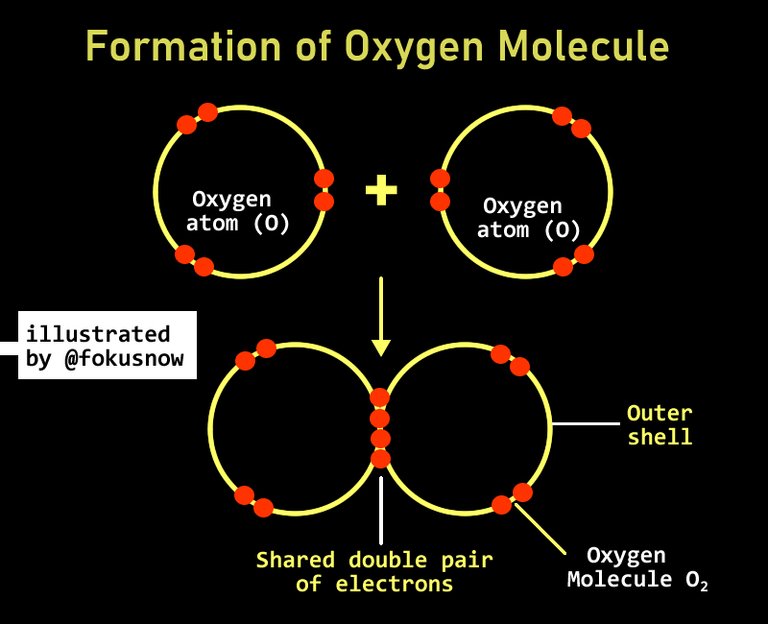
- Double covalent bonds: In double covalent bonding, the two atoms involved in the reaction contribute at least 2 valence electrons or even more than two. Each of the contributed pair is shared by the atoms involved in order to have a complete outer shell. This also results in the formation of molecules. There are many examples, consider the one below.
In the formation of an oxygen molecule, two oxygen atoms combine. They do so by contributing to valence electron each which is then shared to the pair of reacting atoms as shown below:
- Triple covalent bonds: There are some reactions though where the pair of atoms donate 3 electrons and these are shared in the combination. Triple covalent bonds or combinations are very popular among non-metals. Some examples also stand out here. In the formation of Nitrogen molecule, three nitrogen atoms combine and share their valence electrons.
The breaking of the bonds of nitrogen molecule requires a high degree of force, which is why it could be a very dangerous process since a lot of heat is released in the process. That is the reason why the Nitrogen gas is a very stable molecule which does not readily react with other elements in any chemical processes.
Take a good look below at the representation.
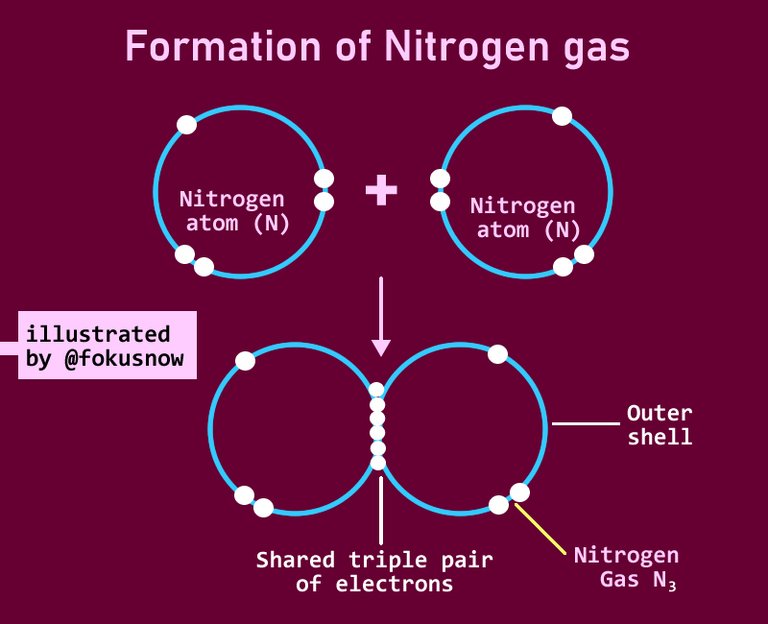
Notice that each nitrogen atom has 5 valence electrons in their outermost shell. They need extra 3 electrons to achieve a stable configuration. In this formation of the Nitrogen gas, each reacting atom shares 3 valence electrons with the other atom. So there are 3 pairs of shared electrons which makes each of the atoms to have a complete total of 8. This then results in the formation of Nitrogen gas.

Characteristics of Covalent Compounds
1. Covalent compounds usually have a three-dimensional shape like crystals.
2. They are not soluble in water. But they can dissolve in non-polar solvents.
3. Covalent compounds are usually gases or volatile liquids.
4 Majority of covalent compounds do not conduct electricity.

Conclusion
Now you have a very good idea of another important type of chemical bonding - covalent bonds. Unlike electrovalent bonds where electrons are transferred, covalent bonds only require the atoms involved to share some electrons. It could be one, two or three electrons.
The general characteristics of covalent compounds have been listed above. I will go deeper into each of these in our next article.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.