ORDINARY MATTER
Solid carbon dioxide, called dry ice because it vaporises at -78 °C without first melting, has being in use as a cleaning agent to strip paint, oil and grime from machinery parts. Equipment like a sandblaster sprays grains of dry ice at the parts. If paint is being removed, the number of layers that come away depends on the strength of the jet. The jet is controlled so that the material underneath will be undamaged.

The dirt is removed in two ways. First, since the temperature of the ice is -78 °C, the dirt cools rapidly and contracts, breaking its contact with the underlying material. Second, the hard grains of carbon dioxide chip away at the grime. Grains manage to pass through the dirt to the surface being cleaned. The impact heats the grains and they vaporise instantly, increasing their volume by 800 times. The gas forms little explosions behind the dirt and so strips it off.
The dry ice evaporates completely in the cleaning process, so it generates no waste, apart from the material it removes. So it merely returns a small amount of carbon dioxide to the environment, in contrast to the use of solvents in more conventional cleaning methods.
The ideas in this chapter
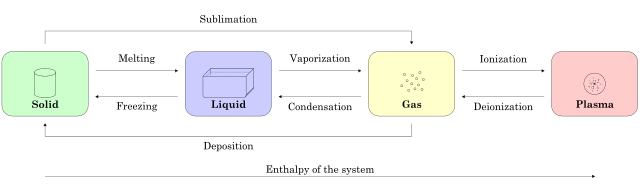
Every day, we see water pouring from a tap or moving in a river. We say it is liquid. Although water keeps the same volume as it moves, it does not keep its shape. When water is cooled below 0°C, it loses its ability to flow and turns to ice. Ice retains its shape as well as its volume. Before the days of refrigerators, blocks of ice were cut in winter and stored in special ice-houses for keeping food cool in summer. The properties of ice are so different from those of water that we say that water changes its 'state of matter' (or 'phase') when it transforms to ice. It has become a solid.
When we heat water in a kettle to 100°C, we get yet another state of matter - it becomes a gas. Most other materials exhibit these different states of matter, although they often do not change their phase under ordinary conditions of temperature and pressure.
There is a fourth state of matter (called plasma) which requires very high temperatures for its existence;
THE STATES OF MATTER
Liquid water is composed of molecules that are constantly moving. During the heating of water in a kettle these molecules begin to move around so rapidly that they leave the kettle and join the molecules of air in the room.
The water is evaporating and becoming a gas. To some extent this would happen to the water even if it were not heated - heating simply speeds up the process. A gas does not keep to a fixed volume – the molecules disperse throughout the room.
Note that the 'steam' you see coming from a boiling kettle is not a gas. In fact, this 'steam' is made up of tiny droplets of water, produced when water vapour (gas) condenses in the cooler air. These droplets are then carried along in the gas flow.
The main difference between the different states of matter is how the individual atoms or molecules are held (bound) together. In a solid they are held so tightly that little movement is possible. The atoms or molecules can vibrate back and forth - the higher the temperature, the more they vibrate. But it is very difficult for them to push past each other. They take up a specific pattern in ice.
In a liquid, the atoms or molecules are attracted but cannot move past each other easily. Water consists of molecules composed of two atoms of hydrogen and one of oxygen, although a minute proportion of the molecules (1 in 107) may separate into oppositely charged particles called ions.
When the water becomes a gas, the gas molecules travel freely at high speeds, as we shall see later. The molecules are spaced wide apart - a gas is nearly one thousand times less dense than a solid.
So, the different states of matter have very different properties. One important property is density, usually labelled p (Greek "rho'). Density is the mass per unit volume. That is, if M is the mass and V is the volume: density = mass / volume
If we look at the densities of common materials at room temperature we should be able to identify their states. In a table that gives this information for a selection of materials, it is observed that there are large differences in density between gases and liquids and between gases and solids. The difference in density between a solid and a liquid is much less clear cut and there are some anomalies (peculiarities). For instance, the density of mercury at room temperature is higher than the density of copper- but mercury is a liquid because it can flow and copper is a solid because it is hard and can be cut. As another instance, glycerol as a liquid has a similar density to a typical plastic.
Most substances are denser as a solid than as a liquid. Yet compare water and ice: at 0 °C ice must be less dense than water, since ice forms on the surface of ponds and icebergs float on the surface of the sea. If the ice in the pond did not form on the surface but sank to the bottom, the entire pond would eventually freeze. We go to considerable lengths to insulate water pipes in winter because water expands as it freezes. When the water becomes ice its volume increases, so it breaks open the pipes. Because the ice cannot flow, a householder knows there is a problem when water doesn't flow-or when the ice turns back to water!
Evaporation
When water evaporates the gaseous molecules have much more energy than the liquid water molecules. Only those molecules with enough energy can escape - that is why heating a liquid increases the rate of evaporation. The rate at which the liquid evaporates can also be increased by increasing the surface area of the liquid: the rate of evaporation is proportional to the surface area. The rate can also be increased by blowing a stream of air across the surface. This reduces the opportunity for the molecules to condense back again.
Evaporation is the method by which the human body controls its temperature. Sweat glands produce water on the surface of the body.
When this water evaporates, energy is taken up by the water molecules and there is less energy left at the surface of the skin. Ultimately this produces a cooling effect on the body. A balance can be created between energy being produced by the body and energy being lost by evaporation such that a constant body temperature is maintained.
CHANGING THE STATE OF A SUBSTANCE
Let us continue to consider water. In order to turn it into ice we must reduce its temperature to 0°C (zero Celsius) and then take away more energy thermally so that freezing begins. The usual method of extracting this heat is to cool the surroundings to below 0°C so that the water loses energy to these surroundings.

The energy that water at 0°C must lose so that it turns to ice at 0°C is called the latent heat of fusion. It is the energy that must be taken away from the molecules to reduce their ability to move around; its removal does not actually go towards reducing the temperature. The temperature at which this takes place is called the temperature of fusion. For water, the temperature of fusion is 0°C. If we go in the other direction, that is, if we warm ice to turn it to water, this critical temperature is called the temperature of melting. Temperatures of fusion and melting are commonly called melting points.
To turn water into its gaseous state, we first heat it to 100 °C, the boiling temperature. We continue heating to provide the latent heat of vaporization. This is the energy we must give to the water molecules to increase their separation and provide them with the kinetic energy they need to achieve the gaseous form. This kinetic energy is large, and the latent heat of vaporisation is greater than the latent heat of melting.
In a figure showing the change in temperature of a substance over time as it is heated at a constant rate through its different phases. During the periods of melting and vaporisation the temperature remains constant. The energy input provides the required latent heat.
The latent heats required to convert 1 kg of some materials from one state to another is called specific latent heat. As latent heat is measured in joules, specific latent heat is measured in joules per kilogram (J/kg).
For a substance of total mass m and specific latent heat L, we shall need an amount of energy E given by:
Energy = mass × latent heat for 1 kg
E = mL
Plasma – a fourth state of matter
If we heat gas molecules to a very high temperature, we obtain a fourth state of matter, a plasma. It has been known for hundreds of years that chemical reactions are not sufficient to produce the energies emitted by stars, including the Sun. In the Sun, temperatures are so high that the outer electrons of the atoms are separated from the protons and neutrons of the nuclei. The resulting positively and negatively charged particles move around separately and rapidly as a plasma.
This is how plasmas form. Because stars consist of large amounts of matter, the gravitational force tends to compress the particles into a smaller volume. The pressure increases and the electrons and protons move faster and faster. The temperature rises further and energy is radiated as electromagnetic rays (photons). Energy can only continue to be emitted if a nuclear reaction takes place. With fusion of the nuclei of elements at the low end of the Periodic Table, a minute proportion of their mass is converted to very large quantities of energy. Thus the high temperature is sustained.
![Lightning as an example of plasma present at Earth's surface: Typically, lightning discharges 30 kiloamperes at up to 100 megavolts, and emits radio waves, light, X- and even gamma rays.[34] Plasma temperatures can approach 30000 K and electron densities may exceed 1024 m−3.](https://images.hive.blog/768x0/https://upload.wikimedia.org/wikipedia/commons/thumb/1/1f/Bliksem_in_Assen.jpg/640px-Bliksem_in_Assen.jpg)
Plasmas do normally occur on Earth, outside the laboratory. A lot of research is carried out on them in the hope of producing large quantities of energy through nuclear fusion for the twenty-first century. It is difficult to produce sufficiently high temperatures to sustain high-energy plasmas (although a plasma is produced by argon-arc welding equipment). In an attempt to create fusion conditions, small capsules of hydrogen or helium are bombarded by laser beams from several directions to create high temperatures that will produce a plasma, and magnetic fields hold the charged particles of the plasma at high density. But sustained nuclear fusion is yet to be achieved.
I’ll be stopping here till next time where I will be discussing the dependence of melting and boiling temperatures on pressure. Till next time, I remain my humble self, @emperorhassy.
Thanks.
REFERENCES
https://en.wikipedia.org/wiki/Dry-ice_blasting
https://www.foodqualityandsafety.com/article/a-cool-way-to-clean/
https://en.wikipedia.org/wiki/State_of_matter
https://www.livescience.com/46506-states-of-matter.html
https://courses.lumenlearning.com/introchem/chapter/three-states-of-matter/
https://en.wikipedia.org/wiki/Evaporation
https://www.britannica.com/science/evaporation
https://www.usgs.gov/special-topic/water-science-school/science/evaporation-and-water-cycle
http://www.chem4kids.com/files/matter_changes.html
https://www.rsc.org/education/teachers/resources/aflchem/resources/16/index.htm
https://www.britannica.com/science/plasma-state-of-matter
https://www.livescience.com/54652-plasma.html
https://en.wikipedia.org/wiki/Plasma_(physics)
http://pluto.space.swri.edu/image/glossary/plasma.html
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @steemstem account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.