Why do we use oil to fry food?
There is no doubt that when it comes to eating, fried food has its place among the culinary guilty pleasures. Who doesn't love golden fries or crispy chicken? We all know well the negative effects that eating food saturated in fat has on our health, and on the other hand, oil suffers different chemical reactions when heated, hydrolysis, caused by the mixture with water and the temperature used for frying, produces the release of free acids that facilitate the formation of carcinogenic substances.

Fried food is a temptation. Composition made by @emiliomoron, using images from pixbay.com: 1, 2, 3.
So, if exposing food to a cooking method that can be harmful to us, and we are aware of this, the obvious question is why do we do it? And the next one would be, is it the only way to achieve that crunchy and delicious taste? Before judging if there are alternatives, let's try to understand why we use oil in the first place.
Why do we use oil for frying?
Surely when you cook you wouldn't think of frying some eggs in water, almost automatically we choose the oil, because that's what everybody knows, that's how it's always been done, so we can see it's a very ingrained cooking technique.

Cooking in oil is a very common technique. Source: pxhere.com, CC0.
Another reason, and the most obvious one, is that fried foods are very delicious, this is because most of the flavors are liposoluble, fat-soluble substances. Another reason is that oil boils at a much higher temperature than water, or rather, because the smoke point (temperature at which fats begin to decompose) is significantly higher than that of water, it allows foods to be cooked at temperatures above 100 °C, making frying a quick method of cooking.
Although it may not seem so, there are several ways to cook at a temperature higher than 100 °C, but the issue of taste is difficult to solve otherwise.
What happens when you fry food?
Technically speaking, frying is the process by which food is cooked by immersion in oil or edible fat at a temperature above the boiling point of water, between 150 and 180°C[1].
The high temperatures that the oil reaches when cooking produces the sudden evaporation of water, which becomes steam and cooks the food, but at the same time this steam seeks to leave and thus the water is transferred from the food to the surrounding oil causing an exchange, since a part of the oil is absorbed by the food and replaces part of the water that was removed, this significantly influences the final characteristics of the food, especially the flavor, color and aroma.
During frying, a heat transfer occurs between the food and the surrounding oil, water vapor creates escape sites through weak points in the food's cellular structure, the exterior dries out and a series of chemical reactions take place that confer organoleptic properties to the final product. The process occurs in four stages described by Gamble et al[2]:
- Initial heating: at this stage the temperature on the surface of the food is raised to the boiling temperature of the water, heat transfer occurs by natural convection.
- Surface heating: at this stage the mechanism of natural convection changes to forced convection and the loss of water vapor prevents the entry of oil. This is also when the formation of the coating layer begins.
- Decreasing speed stage: this is the longest stage and the one where the greatest water loss occurs, it is when the temperature in the center of the food approaches the boiling temperature of the water. Moisture loss remains constant and then decreases due to reduced water content and thickening of the coating.
- Final stage or bubbling point: Here the apparent cessation of moisture loss occurs, possibly due to reduction of water content or decreased heat transfer from the surface to the center of the feed, the latter being the result of the formation of the crust, which is dry and porous.
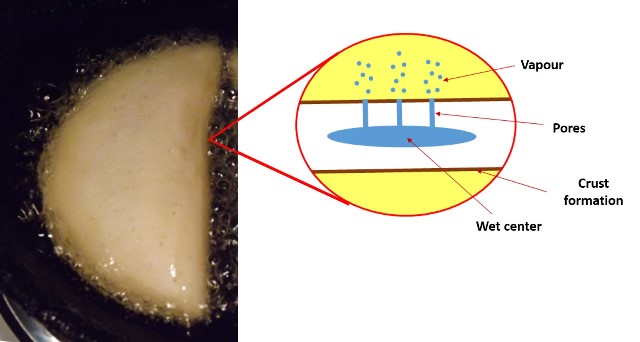
Frying process. Source: @emiliomoron.
When the food is fried other things happen, between 140 and 160 °C a very specific reaction takes place known as Maillard reaction, technically the non-enzymatic glycosylation of proteins, it is a set of very complex chemical reactions that produce colored melanoidins ranging from light yellow to dark brown[3], this reaction is responsible for the brown color and delicious taste of fried food.

Chemical reactions confer that color and flavor when frying food. Source: pxfuel.com.
So, basically the answer to why we fry with oil is given by its physical and chemical properties, which provide the ideal conditions for the right processes and reactions to occur in order to produce a delicious and crunchy dish, since it extracts aromas and other liposoluble substances.
Could we use something else for frying?
Now that we understand what happens when we fry food, the next question to be answered is whether we can use something else to fry that produces the same result. And unfortunately the answer is no.
As we mentioned earlier, the hard thing to reproduce when cooking food differently is the taste. Well, if we want to cook at more than 100 °C using water, we could use some equipment that allows us to increase the pressure enough to make the water reach a higher boiling point, however, we would need the equipment to allow us to introduce the food while maintaining the internal pressure, which would be a bit complicated for a kitchen, but assuming the case, we would manage to cook faster and perhaps manage to form a crispy layer due to the fast evaporation of the water on the surface of the food, but that is without the delicious taste and golden color characteristic of frying in oil.

Even if we could cook with water at more than 100 °C it would still be cooking with steam. Source: Pixabay.com.
In the end, all roads lead to Rome, frying food in oil is the easiest option we have to quickly cook a delicious dish, so oil will still have a reserved place in the kitchen.
In conclusion, the answer to the question why do we use oil for frying? Apart from the obvious fact that frying is very tasty, the answer lies in the formation of that crispy crust that can only be formed when cooking at more than 100 °C, and the set of chemical reactions that determine the color, flavor and taste so characteristic of fried foods.
So in short, we take the risks that come with frying in oil in exchange for a meal with unbeatable taste. So, since we already know that it would simply be very difficult and more expensive to replace the oil for frying, let's just keep in mind some very useful tips:
- Trying not to incorporate too much water when frying, besides being dangerous, causes the hydrolysis of fats, which in addition to producing unpleasant odors leads to an increase in free acids that lead to the formation of other substances dangerous to health.
- Cooking at the right temperature. Wait until the oil is hot, at low temperature the food remains greasy and at high temperature the oil degrades (over 200 °C). At the right temperature the crust of the food cooks quickly and this prevents the absorption of more oil.
- Avoid cutting too thin, if we cut the food very thin or in small pieces the surface per unit of weight is greater, and the greater the amount of oil absorbed.
- Do not reuse the oil too many times. When using fryers, it is advisable to change the oil periodically, as it degrades with each frying, making the food need more cooking time and absorbing more fat[4].
That said, there is nothing more delicious than a piece of fried chicken every now and then.
Well friends, I have found a lot of cloth to cut about the use of edible oils, in other entries I will share other information about these, so I wanted to start by sharing first because we use this element for frying. See you next time!
References
- Wikipedia.com. Fritura
- Montes O., N. (2016). Oil absorption in fried foods. Revista Chilena de Nutrición, vol.43 no.1.
- Wikipedia.com. Maillard reaction..
- OCU.org. (2017). Estudio: Aceite de freír.
This is really interesting. The title did not do justice to the level of interest that I found. Are there proven evidence that fried foods are carcinogenic? I really wish to know. Link me to good peer-reviewed research, if any.
I found the content quite interesting as well. For me the title was also engaging and piqued my interest. Maybe something in parenthesis could have alluded to the greater depth. Really enjoyable content.
Greetings friends, thank you very much for your appreciation of the article. I will consider your advice for the title. Regarding research, allow me to review in more detail, I have read some press releases that cite some research in which it is mentioned that when oil is heated, potentially carcinogenic compounds appear, such as acrylamide, heterocyclic amines and aromatic hydrocarbons, one of them published in the journal The Prostate associates it with an increased risk of prostate cancer.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider supporting our funding proposal, approving our witness (@stem.witness) or delegating to the @stemsocial account (for some ROI).
Please consider using the STEMsocial app app and including @stemsocial as a beneficiary to get a stronger support.