REDOX reactions of cupric sulfate with various metals
In every chemical reaction there is an interaction between the species that constitute the reactants, there are many ways in which these species can interact, either by decomposing, combining, displacing each other, and among this set of forms interaction, the so-called oxidation-reduction reactions, also called REDOX are very important in chemistry.
A REDOX reaction is produced by an exchange of electrons, one of the reactants gives up or loses electrons and the other reactant accepts or receives them; in this process, the reactant that loses electrons is the one that is oxidized and the one that receives them is said to be reduced. To illustrate this type of chemical reactions we are going to see in this post some experiments of oxidation-reduction between cupric sulfate (CuSO4) and several metals.

We will test the reaction between a solution of cupric sulfate and various metals. Source: @emiliomoron.
Cupric sulfate
Cupric sulfate or also copper (II) sulfate, chemical formula CuSO4 in its anhydrous form, is a compound widely used in the chemical industry, it is used in agriculture as a pesticide, it also has application in water treatment as an algaecide; the most common way to find this compound is in its pentahydrate form, CuSO4x5H2O, which in its crystallized salt form is blue, so it gives us solutions of this color, and in solution, it is used as an electrolyte to produce multivalent cations: Cu2+, which is why it is widely used in the metal coating industry by electrolytic means.

Cupric sulfate pentahydrate (left) and anhydrous cupric sulfate (right). Source: @emiliomoron.
Experiments
To demonstrate the concept of electron exchange that takes place in REDOX reactions, we are going to put the metals aluminum, iron and zinc in direct contact with a solution of cupric sulfate. As we will see, the metals we are going to use are less noble than copper according to their reduction potentials, that is why we will see that the Cu2+ ions in solution are transformed into metallic copper at the cost of oxidizing the less noble metals that enter the solution.

Materials to be used in the experiment. Source: @emiliomoron.
Reaction between cupric sulfate and aluminum
For this first experiment we are going to immerse an aluminum foil in a solution of cupric sulfate.

Beginning of the experiment. Source: @emiliomoron.
After immersing the film in the solution we will observe a bubbling and the deposition of a reddish solid on the film.

Experiment progress. Source: @emiliomoron.
The reddish deposit we observe is due to the oxidation of metallic aluminum to Al3+ ions and the reduction of Cu2+ ions to metallic copper.

Result of the experiment. Source: @emiliomoron.
The deposition of copper on the aluminum foil is due to the following half-reactions:
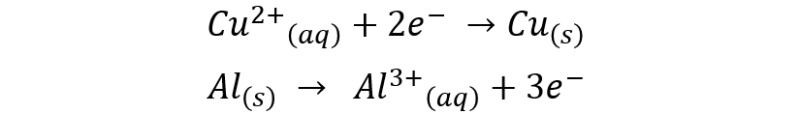
The global reaction can be represented as:

This is why when the aluminum foil is immersed in the cupric sulfate solution, a reddish layer forms on the foil, which corresponds to the reduced copper deposited on the aluminum, while aluminum ions are transferred to the solution due to the loss of electrons.
On the other hand, the bubbling is due to the release of hydrogen according to the reaction:
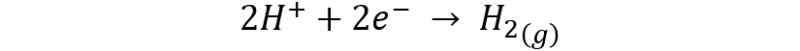
Reaction between cupric sulfate and iron
Let's see how this situation is represented but when introducing an iron nail in the solution of cupric sulfate.

Start of the experiment. Source: @emiliomoron.
In a similar way to the previous case, when the iron nail is introduced into the solution we will notice that in a few moments it begins to be covered with a reddish layer, again it is the reduced copper deposited on the iron.

Experiment progress. Source: @emiliomoron.
The process is described by the following reactions:
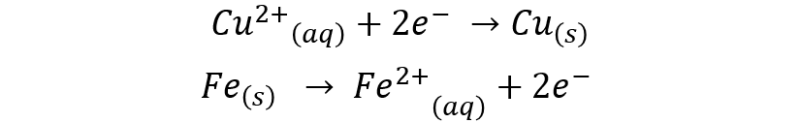
The global reaction would be represented as follows:
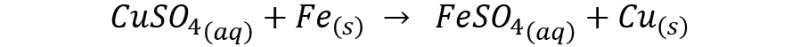
In the following image we can observe the deposition of copper on the submerged part of the nail.

Result of the experiment. Source: @emiliomoron.
Reaction between cupric sulfate and Zinc
We will proceed in the same way as in the previous cases, now immersing in the solution a zinc sheet that I have extracted from a worn out battery.

Beginning of the experiment. Source: @emiliomoron.
In this case we will notice that just seconds after having submerged the zinc sheet in the solution it acquires a black coating, it is also metallic copper that is reduced by the acceptance of electrons is deposited on the zinc, only in this case the deposition is very fast and the copper does not crystallize properly with that characteristic reddish color. On the other hand, zinc loses two electrons and goes into solution in the form of zinc ions.

Experiment progress. Source: @emiliomoron.
The process is described according to the following half-reactions:
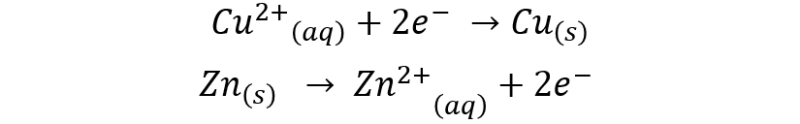
The global reaction would be as follows:

In the following image we can see the result of the copper deposition on the zinc foil.

Result of the experiment. Source: @emiliomoron.
In the following image we can compare the copper coating obtained on the different metals by means of an exponential redox reaction.

Result of the REDOX reaction between cupric sulfate and the metals used in the experiment. Source: @emiliomoron.
As we can see, it is easy to interpret a chemical reaction when it is accompanied by a demonstrative experience, in this case, it is easy to visualize how the copper that is in the form of ions in the solution is able to deposit itself on less noble metallic elements such as zinc or iron, and with this we can demonstrate how this effect occurs due to the transfer of electrons in what constitutes a REDOX reaction.
Well friends, I hope you liked the experiments and that you have shared interesting information about this important type of chemical reaction. See you next time!
References
Wikipedia.com. REDOX.
Atkins, P., Jones, L. (2006). Principios de química. 3a edición, Médica Panamerica, Buenos Aires.
This was really a cool post. I really enjoyed the illustrations of the experiments that you conducted. That's in my opinion the best way to teach science to the younger ones in a way they will remember: experimenting by themselves!
Cheers!
Thank you very much @lemouth, I'm glad you liked the post and the illustrations, and I totally agree with you, the best way to teach science is through experiences in which students can experience and evidence for themselves the phenomenon of interest.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.
Thank you very much for your support my friends!