From carbon dioxide to methane

If we cannot avoid the generation of CO2 we can recycle it. Source: Edited image, original from pixabay.com.
But despite the advances in obtaining clean energy, there is still a long way to go to break our dependence on hydrocarbons, so one of the strategies that could help us mitigate CO2 emissions is to recycle it and use it to manufacture synthetic fuels.
Among the products that can be obtained from CO2, conversion to methane is one of the processes that is currently of greatest interest. This process is known as methanation, a chemical reaction in which carbon dioxide combines with hydrogen in the presence of a catalyst to form methane and water as products.
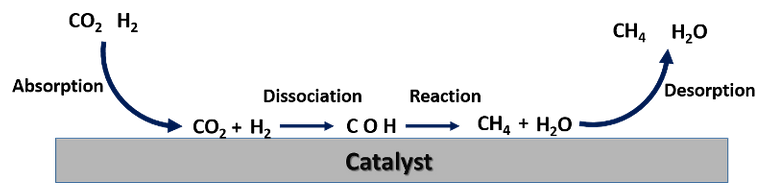
The methanation reaction can be represented as follows. Image elaborated in Powerpoint.
In this regard, a team of researchers has recently published in the journal Angewandte Chemie that they have succeeded in synthesizing a catalyst based on nickel and carbon that has shown excellent activity for the photothermal conversion reaction of CO2.
A photothermal process is a chemical process in which the action of light and heat is combined, and is proving to be a promising method to valorize different products such as CO2. Although different catalysts have been explored for methanation, mainly based on palladium and ruthenium, this team attempted to use a cheaper metal for the synthesis of a catalyst, opting for nickel, which although it has already been used for this reaction, it has been found that its performance is affected by the type of support, rapidly losing its activity.
But to improve the process, and replace expensive metals with a more abundant one, they opted to use a high loading of nanoparticles on a carbonaceous support, since materials such as graphene and carbon nanotubes have shown good qualities as supports for photothermal catalysts due to their good light absorption.
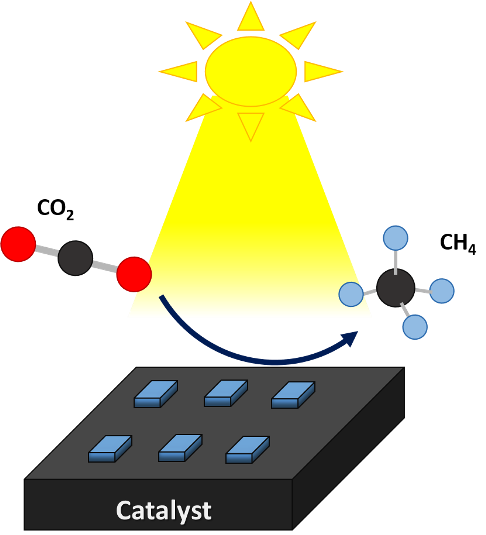
Representation of the conversion of CO2 to methane on the surface of the catalyst. Image elaborated in powerpoint.
To synthesize the catalyst, the team used an organometallic compound containing nickel, and subjected it to a controlled pyrolysis process at various temperatures, finding that the best synthesis temperature was 600 °C, to obtain very uniform nickel nanoparticles, well distributed and embedded in the porous matrix of the graphitic carbon.
The obtained catalyst, called Ni@C, was tested in the reduction of CO2 to methane, demonstrating a high conversion rate for methanation, under artificial UV light and natural sunlight, during the process, the catalyst remained stable for more than 12 hours.
This type of research shows us that there is a promising technology that would allow us to obtain an energy source from CO2 and hydrogen. This would allow us to have hydrocarbons without having to add more greenhouse gases to the environment, only the gases generated in power plants or other fixed sources of CO2 generation would be recycled.
Therefore, photothermal CO2 methanation technology looks like a sustainable alternative to mitigate the adverse effects of the accumulation of this gas in the atmosphere, and although it is still under study, it is already generating great interest due to its potential chemical storage of energy in the form of methane. Hopefully, the development of catalysts, and of the technology in general, will soon scale up to conditions where it can be used industrially and provide us with a valuable gas that can be used in heating, electricity generation and industry.
Thanks for coming by to read friends, I hope you liked the information. See you next time.