Explaining Chemistry In Simple Words (Bonding and Electronegativity)
Today, we are continuing our chemistry class. Remember that in my previous post, I discussed different elementary things ranging from Atoms, Ions, Molecules, and Compounds. If you missed the post, you can check out Explaining Chemistry In Simple Words (Part 1). That said, let's continue with our chemistry learning.
There are several ways to write molecules, as we count the number of atom in each molecule, and write the atom as a subscript number next to the symbol of the element such as in the case of Hydrogen (H2) and Oxygen (O1) to become H2O but this can lead to another problem where two molecules can have the same molecular formula but they are not the same. This is called Isomers and this is what differentiates Diamonds from Graphite because they have the same carbon allotropes.
For ease of identifying elements even when they have the same molecular formula but different properties is the Lewis dot Structure. With Lewis dot structure, those bonds and valence electrons are represented as dots and lines. With atoms, the state of their lowest potential energy is when they have a full outer shell of electron which can be 2 in the case of hydrogen (H) and Helium (He), or 8 in the case of Neon (Ne) Oganesson (Og), or Argon (Ar).
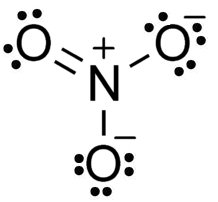
wikimedia
When atoms have their full outer shell, they do not want to react with anything and we see this with the case of all the noble gases. When two atoms do not have a full outer shell but can achieve it by sharing electrons, then they do so. When they do so, it is known as covalent bonding and they are referred to as a covalent bond where the positive charge of a nucleus is pulling the electron (-) of another atom and we have a name for that strength and it is Electronegativity and on the periodic table, the strength of electronegativity increases from the bottom left to the top right on the periodic table and with this, it means that fluorine has the strongest electronegativity.
When pure metals bond, they form metalic bond, and a bonding between metals and non-metals give rise to a salt. The positive nuclei in metals are free to move which is why the valence electron in metals are delocalized (move freely) and this is what gives them their conducting characteristics of heat and electricity as well as being malleable.
Let's talk about solubility and to discuss this, let's look at the polarity of water that makes it the most versatile solvent in the world and this polarity allows it to be able to pull apart any molecule by holding on to one charge thereby keeping them apart by surrounding the particle with its oppositely charged end but this works for polar molecules but it cannot dissolve non-polar molecules and this is why the oil in your kitchen will never mix with oil and this is because fat molecules are not polar. Now if we are going to rank the bonds in chemistry by strength we can start with ionic bonds, covalent bond, metalic bonds, hydrogen bonds, and van der wall force.
Reference
https://chem.libretexts.org/Bookshelves/General_Chemistry/
https://chem.libretexts.org/Courses/Bellarmine_University/BU%3A_Chem_103
https://chemistrytalk.org/electronegativity-chart-trends
https://chem.libretexts.org/Courses/Northern_Michigan_University
https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental
https://chem.libretexts.org/Bookshelves/Organic_Chemistry
https://www.masterorganicchemistry.com/2018/09/10/types-of-isomers/

Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
Thanks for including @stemsocial as a beneficiary, which gives you stronger support.