Physics - Classical Mechanics - Density and Pressure
[Image1]
Introduction
Hey it's a me again @drifter1!
Today we continue with Physics, and more specifically the branch of "Classical Mechanics", in order to start with a new chapter : "Fluid Mechanics". This article will be a small introduction to Fluids, and Density and Pressure.
So, without further ado, let's get straight into it!
Fluids and Fluid Mechanics
In Physics, any substance that "flows" is considered a Fluid. Thus, both the liquid and the gas state of matter is considered a fluid.
The study of the motion of such fluids is known as Fluid Mechanics. This branch of Physics can further be divided into Fluid Statics, which studies fluids at rest, and Fluid Dynamics, which studies fluids in motion.
The first sub-branch covers simpler concepts such as density, pressure, and Pascal's and Archimedes' principles, whilst the second one is one of the most complicated branches of Mechanics! Therefore, we will only get into concepts that are based on idealized models (such as Newton's Laws and Conservation of Energy), which are easier to comprehend.
Density
Before we can get into the more interesting topics, we first have to understand the basics. Let's start off with density...
Density is a characteristic of substances, which is defined as the mass per unit volume. It's denoted by the Greek letter ρ. The S.I. unit of density is the Kg / m3.
So, mathematically, density can be expressed as:
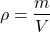
The density isn't always constant throughout the volume of a substance. Only a homogeneous substance has the same density across it's volume.
Local density can be obtained using a limit, where the volume approaches zero:
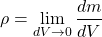
The Effects of Temperature and Pressure
The density of gases is much less than the density of liquids and solids. Additionally, density is also affected by temperature. In the case of gases it's also affected by pressure.
The densities of gases are commonly displayed at standard temperature (0° C) and standard pressure (101.3 kPa), whilst solids and liquids are displayed at standard temperature (0° C). The density usually increases with the decrease of temperature.
Specific Gravity
In order to make it easier to compare the density of various substances, which is a dimensional property (units must be taken into consideration), we define a quantity which is dimensionless. This quantity is known as specific gravity, and is defined as the ratio of the density of the substance by the density of water at 4° C and 1 atm of pressure. The density of water at these conditions is exactly 1000 Kg / m3 or 1 g / cm3.
For example, in the case of aluminum, which has a density of 2.7 g / cm3, its specific gravity is 2.7 which is much easier to work with.
Pressure
Next up is the topic of pressure...
Pressure is the normal force per the unit area over which the force is applied. So, it's the force perpendicular to the area divided by the area itself:
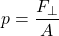
The pressure at a specific point is defined as:

Pressure is a scalar and not a vector unit. The S.I. unit is the pascal (Pa), which is a shorter way of saying N / m2. Other common units are the bar, which equals 105 Pa and the millibar, which equals 100 Pa.
Atmospheric Pressure
Atmospheric pressure, pa, is the pressure exerted by the atmosphere of the Earth. Its value depends on the weather, height etc.
A standard atmosphere, denoted by atm, is a unit of pressure, which equals about 1.013 x 105 Pa = 1.013 bar.
RESOURCES:
References
- https://en.wikipedia.org/wiki/Fluid_mechanics
- https://courses.lumenlearning.com/suny-osuniversityphysics/chapter/14-1-fluids-density-and-pressure/
Images
Mathematical equations used in this article, where made using quicklatex.
Visualizations were made using draw.io.
Previous articles of the series
Rectlinear motion
- Velocity and acceleration in a rectlinear motion -> velocity, acceleration and averages of those
- Rectlinear motion with constant acceleration and free falling -> const acceleration motion and free fall
- Rectlinear motion with variable acceleration and velocity relativity -> integrations to calculate pos and velocity, relative velocity
- Rectlinear motion exercises -> examples and tasks in rectlinear motion
Plane motion
- Position, velocity and acceleration vectors in a plane motion -> position, velocity and acceleration in plane motion
- Projectile motion as a plane motion -> missile/bullet motion as a plane motion
- Smooth Circular motion -> smooth circular motion theory
- Plane motion exercises -> examples and tasks in plane motions
Newton's laws and Applications
- Force and Newton's first law -> force, 1st law
- Mass and Newton's second law -> mass, 2nd law
- Newton's 3rd law and mass vs weight -> mass vs weight, 3rd law, friction
- Applying Newton's Laws -> free-body diagram, point equilibrium and 2nd law applications
- Contact forces and friction -> contact force, friction
- Dynamics of Circular motion -> circular motion dynamics, applications
- Object equilibrium and 2nd law application examples -> examples of object equilibrium and 2nd law applications
- Contact force and friction examples -> exercises in force and friction
- Circular dynamic and vertical circle motion examples -> exercises in circular dynamics
- Advanced Newton law examples -> advanced (more difficult) exercises
Work and Energy
- Work and Kinetic Energy -> Definition of Work, Work by a constant and variable Force, Work and Kinetic Energy, Power, Exercises
- Conservative and Non-Conservative Forces -> Conservation of Energy, Conservative and Non-Conservative Forces and Fields, Calculations and Exercises
- Potential and Mechanical Energy -> Gravitational and Elastic Potential Energy, Conservation of Mechanical Energy, Problem Solving Strategy & Tips
- Force and Potential Energy -> Force as Energy Derivative (1-dim) and Gradient (3-dim)
- Potential Energy Diagrams -> Energy Diagram Interpretation, Steps and Example
- Internal Energy and Work -> Internal Energy, Internal Work
Momentum and Impulse
- Conservation of Momentum -> Momentum, Conservation of Momentum
- Elastic and Inelastic Collisions -> Collision, Elastic Collision, Inelastic Collision
- Collision Examples -> Various Elastic and Inelastic Collision Examples
- Impulse -> Impulse with Example
- Motion of the Center of Mass -> Center of Mass, Motion analysis with examples
- Explaining the Physics behind Rocket Propulsion -> Required Background, Rocket Propulsion Analysis
Angular Motion
- Angular motion basics -> Angular position, velocity and acceleration
- Rotation with constant angular acceleration -> Constant angular acceleration, Example
- Rotational Kinetic Energy & Moment of Inertia -> Rotational kinetic energy, Moment of Inertia
- Parallel Axis Theorem -> Parallel axis theorem with example
- Torque and Angular Acceleration -> Torque, Relation to Angular Acceleration, Example
- Rotation about a moving axis (Rolling motion) -> Fixed and moving axis rotation
- Work and Power in Angular Motion -> Work, Work-Energy Theorem, Power
- Angular Momentum -> Angular Momentum and its conservation
- Explaining the Physics behind Mechanical Gyroscopes -> What they are, History, How they work (Precession, Mathematical Analysis) Difference to Accelerometers
- Exercises around Angular motion -> Angular motion examples
Equilibrium and Elasticity
- Rigid Body Equilibrium -> Equilibrium Conditions of Rigid Bodies, Center of Gravity, Solving Equilibrium Problems
- Force Couple System -> Force Couple System, Example
- Tensile Stress and Strain -> Tensile Stress, Tensile Strain, Young's Modulus, Poisson's Ratio
- Volumetric Stress and Strain -> Volumetric Stress, Volumetric Strain, Bulk's Modulus of Elasticity, Compressibility
- Cross-Sectional Stress and Strain -> Shear Stress, Shear Strain, Shear Modulus
- Elasticity and Plasticity of Common Materials -> Elasticity, Plasticity, Stress-Strain Diagram, Fracture, Common Materials
- Rigid Body Equilibrium Exercises -> Center of Gravity Calculation, Equilibrium Problems
- Exercises on Elasticity and Plasticity -> Young Modulus, Bulk Modulus and Shear Modulus Examples
Gravity
- Newton's Law of Gravitation -> Newton's Law of Gravity, Gravitational Constant G
- Weight: The Force of Gravity -> Weight, Gravitational Acceleration, Gravity on Earth and Planets of the Solar System
- Gravitational Fields -> Gravitational Field Mathematics and Visualization
- Gravitational Potential Energy -> Gravitational Potential Energy, Potential and Escape Velocity
- Exercises around Newtonian Gravity (part 1) -> Examples on the Universal Law of Gravitation
- Exercises around Newtonian Gravity (part2) -> Examples on Gravitational Fields and Potential Energy
- Explaining the Physics behind Satellite Motion -> The Circular Motion of Satellites
- Kepler's Laws of Planetary Motion -> Kepler's Story, Elliptical Orbits, Kepler's Laws
- Spherical Mass Distributions -> Spherical Mass Distribution, Gravity Outside and Within a Spherical Shell, Simple Examples
- Earth's Rotation and its Effect on Gravity -> Gravity on Earth, Apparent Weight
- Black Holes and Schwarzschild Radius -> Black Holes (Creation, Types, How To "See" Them), Schwarzschild Radius
Periodic Motion
- Periodic Motion Fundamentals -> Fundamentals (Period, Frequency, Angular Frequency, Return Force, Acceleration, Velocity, Amplitude), Simple Harmonic Motion, Example
- Energy in Simple Harmonic Motion -> Forms of Energy in SHM (Potential, Kinetic, Total and Maximum Energy, Maximum Velocity), Simple Example
- Simple Harmonic Motion Equations -> SHM Equations (Displacement, Velocity, Acceleration, Phase Angle, Amplitude)
- Simple Harmonic Motion and Reference Circle -> SHM and Smooth Circular Motion, Reference Circle
- Simple Harmonic Motion Exercises -> 2 Complete Examples on Simple Harmonic Motion
- Simple Pendulum -> Simple Pendulum (Return Force, Small Angle Approximations, More Accurate Period, Gravity Approximation)
- Physical Pendulum -> Physical Pendulum (Return Torque, Small Angle Approximations, Estimating Moment of Inertia)
- Exercises around Pendulums -> Complete Examples on the 2 types of Pendulums (Simple, Physical)
- Damped Oscillation -> Damping Force, Total Force and Differential Equation, Motion Equations, Special Cases
- Forced Oscillation and Resonance -> Forced Oscillation (Differential Equation, Amplitude, Resonance)
- Exercises around Damped and Forced Oscillation -> Complete Examples on Damped Oscillation and Forced Oscillation
- Chaos and Chaotic Oscillation -> Chaos, Unpredictability and Randomness, Chaotic Oscillation
Final words | Next up
And this is actually it for today's post!
Next time we will get more in depth into how we measure the pressure in fluids.
See ya!

Keep on drifting!
Posted with STEMGeeks
Electronic-terrorism, voice to skull and neuro monitoring on Hive and Steem. You can ignore this, but your going to wish you didnt soon. This is happening whether you believe it or not. https://ecency.com/fyrstikken/@fairandbalanced/i-am-the-only-motherfucker-on-the-internet-pointing-to-a-direct-source-for-voice-to-skull-electronic-terrorism
I wonder how much this relates to using say a shirt press with a pneumatic press to squeeze some cannabis. The plant is squeezed so hard with the low heat of the shirt press melts out the oil, would that be considered a liquid... Interesting blog! !1UP
You have received a 1UP from @dynamicrypto!
@stem-curatorAnd they will bring !PIZZA 🍕
Learn more about our delegation service to earn daily rewards. Join the family on Discord.