What Is The Hardest Natural Substance?
Corundum, which is just 30% as hard as diamond, is the next hardest material known to science after diamond. This makes diamond an extremely hard substance with a Vickers hardness rating between 70 and 150 GPa. Because of this crucial characteristic, diamonds are employed to manufacture the cutting ends of machines used to cut a variety of materials. For example, in a microtome, diamond-cutting blades are incorporated to cut extremely thin slices of teeth, bones, and plants for microscopic analysis.
A diamond knife blade used for cutting sections for transmission electron microscopy.

Pyramidal diamond anvil impactor on a Vickers hardness tester
Turning Graphite into Diamond:
Because diamonds are rarely found in nature, they are produced artificially under specific temperature and pressure conditions. At temperatures close to 3,000 °C and pressures of about 125,000 atm, graphite transforms into diamond. By employing a catalyst such as platinum, nickel or iron, the pressure required to turn graphite into diamond can be decreased to between 60,000 and 80,000 atm. Diamonds created artificially are solely utilised for industrial purposes and lack the beauty of gemstones.
The diamond's hardness and crystal structure:
The melting point of diamond is quite high, around 3550 °C. But when heated to 900 °C, it burns in the air and vanishes as carbon dioxide gas, leaving just a tiny bit of ash behind.
The crystalline structure of diamond is responsible for both its great hardness and high melting temperature. It has four covalent bonds connecting each carbon atom to four other atoms. Typically, diamonds have a tetrahedral structure, with the overlapping sp3 orbitals holding the atoms tightly together.

Diamond cubic crystal structure
Electronic components can be made thanks to diamond's semiconductor qualities!
Since carbon is a semiconductor when it takes the form of a diamond, both valence and conduction bands are separated by a bandgap. Band gap is a measure of the minimum energy needed to break an electron from a bond. When compared to silicon, diamond provides an extremely rare blend of thermal and electronic qualities:
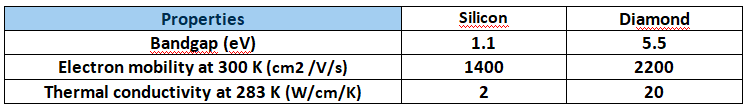
Table: Electrical and thermal properties of diamond compared with silicon.
References:
- Chris J.H. Wort, Richard S. Balmer, Diamond as an electronic material, Materials Today, Volume 11, Issues 1–2, 2008, Pages 22-28, ISSN 1369-7021
- Dang, Chaoqun; et al. (January 1, 2021). "Achieving large uniform tensile elasticity in microfabricated diamond". Science. 371 (6524): 76–78
- [General and inorganic chemistry book- M. Shkhashirou- H. Birqdad- Y. Qodsi- University publications. Algeria]
- [Modern Chemistry, L.Nikolaiev]
- [ Kobashi, Koji (2005), "2.1 Structure of diamond", Diamond films: chemical vapor deposition for oriented and heteroepitaxial growth, Elsevier, p. 9, ISBN 978-0-08-044723-0.]
Corundum is rather rare, not as common as diamonds, gold, and others that we normally know and hear about. Thanks for sharing this information with us.
You're welcome, yes, corundum is not common, but it is just the crystalline form of aluminium oxide with some metal impurities.
Thank you and have a nice day!