Water Treatment "Part 34": Elimination of fluorine and arsenic.
1- Removing fluorine from water:
The majority of fluorine removal techniques used in the process of treatment of drinking water depends on precipitation or adsorption. However, because the procedure gets expensive when there are significant amounts of fluorine, it is best to replace the water source or add another one that is fluoride-free to it.
Precipitation method:
Calcium fluoride precipitates are created when calcium chloride or quicklime is applied to remove fluoride ions.
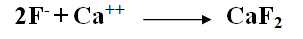
[Made using "MS word" and "Paint"]
It should be noted that the solubility of calcium fluoride is affected by the pH of the medium as well as the overall salinity.
Aluminium salts are also good at precipitating fluorine. The hydrolysis of aluminium sulphate produces aluminium hydroxide, which creates a colloidal phase on which fluoride ions are attached and removed from water by filtering.Adsorption method:
It uses activated alumina or adsorbents composed of bone or wood, and is the most efficient approach.
2- Removing arsenic from water:
Oxidation and precipitation:
To remove arsenic, many oxidants can be utilized, each with advantages and disadvantages as well. Pentavalent arsenic, which is less soluble in water and may be separated by precipitation, is created by oxidizing trivalent arsenic.
When calcium or magnesium are present in water, quicklime can be employed to precipitate pentavalent arsenic, which precipitates with calcium or magnesium carbonate in an alkaline medium (PH= 10.5). Such a solution is viable when the treatment comprises the step of eliminating hardness with quicklime, this approach is not practical in the event of extremely low salinity and low calcium content.Specific adsorption method:
The adsorption process may be carried out on a variety of adsorbents.
Table- 4 lists the most common ways for removing arsenic:

Table- 4: the most common ways for removing arsenic.
[Made using "MS word" and "Paint"]
References:
- [Introduction to Water Chemistry (Pollution- Treatment- Analysis). Dr. Nasser Al-Hayek. Publication of the Higher Institute for Applied Sciences and Technology (HIAST). Syrian Arab Republic, 2017.]
- Taparhudee, Wara (2002). "Applications of Paddle Wheel Aerators and Diffused-Air System in Closed Cycle Shrimp Farm System" (PDF). Witthayasan Kasetsart (Sakha Witthayasat). 36: 408–419. Retrieved 26 April 2020.
- Unsafe water kills more people than war, Ban says on World Day". UN News. 22 March 2010. Retrieved 10 May 2018
- Raymond Desjardins- Livre: Le traitement des eaux- 2éme edition- Ecole Polytechnique de Montréal- 1997- ISBN 2-553-00643-8
- Drinking Water Treatment- EDX- Delft University of Technology.
- Book- Drinking Water: Principles and Practices- by Hans J C Van Dijk (Author), Jasper Q J C Verberk (Author), Peter J De Moel.
Thanks for your contribution to the STEMsocial community. Feel free to join us on discord to get to know the rest of us!
Please consider delegating to the @stemsocial account (85% of the curation rewards are returned).
You may also include @stemsocial as a beneficiary of the rewards of this post to get a stronger support.