Saxon's Survival Hour #187: The Super Still
Today's excerpt begins on page 96 of The Survivor Volume 1.

With the natural pollution of the rural groundwater from sewage, insecticides, herbicides, inorganic minerals and salts form the earth itself, even the back to the landers need a still.
The city dweller, however, is actually in danger without it.
Most city water is fluoridated.
Sodium fluoride is put into everyone's drinking water to protect the teeth of children after they've lost their baby teeth and until their permanent teeth are developed.
Thus, it is useful to children only from about the ages of five to seven.
It may be harmful to children's brains and it is certainly of no value to adults.
I have had experience with fluoride which proves to me that it dulls the creative faculties of the brain.
It doesn't diminish intelligence, but it does block out creativity so a person on a new project can feel rather stupid, helpless and frustrated when trying to get his act together.
A still was tested by the Food, Chemical and Research Laboratories, Inc. in Seattle Washington.
The test was made on tap water containing .92 parts per million of sodium fluoride.
After distillation, the fluoride was less than .05 ppm.
A second laboratory distilled water with 9ppm fluoride.
After distillation no trace of fluoride was detected, again less than .05ppm.
It also leaves behind chemicals and salts commonly found in city water such as chlorine, mercury, sulfates of carbonates, arsenic, sodium, potassium, phosphorus, silicon, calcium, magnesium, chlorides, cyanide, chromium, lead, silver, cadmium, sulfur, nitrates, zinc, iron, copper, phenols, pesticides and herbicides.
These inorganic minerals are not needed by the body.
As a general rule, inorganic minerals can't be utilized by the body until they have been processed by vegetation.
Whether such chemicals, unprocessed by vegetation, come from springs, streams, wells, or the city faucet, they are only excess baggage, at best, and harmful at worst.
They must be flushed out of the system lest they cause general debility or even such things as birth defects, mental disorder, heart trouble, cancer, etc.
Also the already overloaded system cannot be properly flushed out with more polluted water.
Aside from health considerations, there is always the danger of a water cutoff.
In such an event you could gel water from various places but it wouldn't be fit to drink.
Boiling it, treating it with clorox or halazone tablets would spare you only from living organisms.
A city's water supply comes from their best source.
It has less pollution in it than any water you might find outside the water system in that immediate area.
So although you might kill the microrganisms in standing water, it might be filled with lethal doses of pesticides, herbicides, or industrial chemical wastes no one considered removing since that water was never meant for human use, anyway.
That’s why a still could save your life.
In storing water, it is best to store distilled water.
First, there are no living organisms in it.
If some get in after distilling, there is so little mineral content to feed on that they could not thrive.
Ladies will appreciate this still for the completely soft water it produces.
It is perfect for washing the hair and for doing fine washables which break down so quickly when washed with common tap water.
Soft water is also best for washing the face, especially before putting on makeup.
Although the still operates over any heat source, even a camp fire, it does give off some heat of its own.
This is cosy and an energy saver in winter.
But in summer you might want to put it in a back room.
Distilled water is sold in stores for from 40c to $1.50 per gallon for irons, car batteries, photo developing, drinking, etc.
My still makes it for from three to five cents per gallon.
If you do it on a wood stove, it’s free.
Coffee, tea, alcoholic drinks and food taste better when prepared with distilled water.
Also, chemists, and even alchemists, will appreciate the ability to distill the same water several, or even hundreds of times for goodies requiring multi distilled water.
In using this still, too much heat will cause the liquid to boil beyond the coil's capacity to distill it.
This will cause vapor to come out the tube.
You must turn it down then because this means the liquid is escaping and you will not only waste liquid but get less out of the still, since the vapor is going elsewhere.
Cheap wine, and of course, any homemade wine or whisky, will have fusel oils which taste bitter.
It can even be harmful in large quantities.
Dick's 1872 process for removing fusel oil from alcohol.
No. 1445. follows:
"To Free Alcohol from Fusel Oil.
This may be effected by digesting the alcohol with charcoal.
The alcohol is filtered through alternate layers of sand, wood charcoal, boiled wheat and broken oyster shells; this removes all other impurities as well.
The fusel oil can be extracted from small quantities of alcohol, by adding a few drops of olive oil to the spirit, agitating thoroughly in a bottle, and after settling, decanting.
The olive oil dissolves and retains the fusel oil."
For the real poop on alcohol for its own sake, get GRANDDAD S WONDERFUL BOOK OF CHEMISTRY.
Alcoholmetry starts on page 129 and anything else you want to know about it starts on page 134.
From my bottle of Red Mountain Burgundy, I got almost a quart of about 100 proof alcohol for $2.99 plus tax.
A fifth of 100 proof Vodka costs $6.10 plus tax.
This cheap wine has 12% pure alcohol.
That means it has 24%, or almost a quart, of 100 proof alcohol after distillation.
If you cut the 100 proof goody with orange juice or something, you can get just as merry on half or less, since stronger stuff does it better than wine.
This, in itself, would cut your liquor bill by half or more.
(I wouldn't worry about fusel oil in commercial wines or even in the home made stuff.
But if you are into making your own whiskey from mash, you ought to consider filtering it.)
If you want the alcohol for chemistry or making extracts, hash oil, flavors, etc., you can re distill the alcohol until you've got the purest stuff going.
The still would work just as well with whiskey mash.
You could fit the still to a cooker of unlimited size and get at least eight gallons of alcohol in 24 hours since alcohol distills faster than water.
There are several manufactured stills on the market, usually through health food stores.
These are quite expensive so I wanted one anyone could make cheaply.
I designed a heavy duty still and had Clyde Barrow build it.
It didn't work well so he redesigned it and now has an improvised still which works as well as any commercial still on the market.
The Saxon-Barrow, or Barrow Saxon, design is high yield production still.
It processes at least a quart of water per hour and even more alcohol in the same time.
The entire unit can be made for about $25.00 and assembled with simple hand tools.
The unit is easily disassembled for storage.
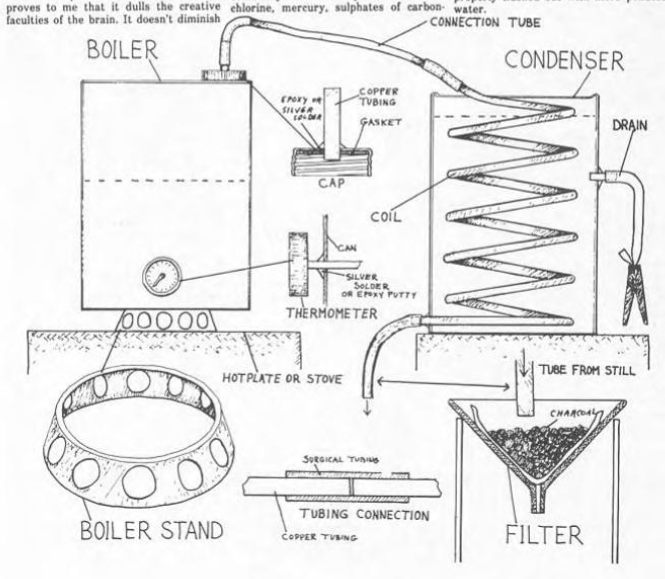
The still has three basic parts.
The part that goes on the fire is the boiler.
We used a five-gallon, square can with a screw-top.
These cans can be bought empty and unused for about $3.00 at paint stores and elsewhere.
You can also buy a two-gallon, unused gas can from any auto supply store.
Either size is cheap enough new that you should avoid trying to use a can which had paint, paint thinner, gas, etc. in it before.
A used can may not be dangerous, but what it had in it could give off volatile residues which will cause a taste in the water or booze for the life of the can.
Begin by drilling, or punching, a hole in the screw-top lid large enough to insert a two inch long copper tube with an outside diameter of 3/8ths of an inch.
Such copper tubing can be bought from most hardware stores for about 59 cents per foot.
Remove the lid’s gasket and epoxy or silver-solder the tube in place.
(See drawing)
When the tubing is firmly set, replace the gasket.
(If you use solder, make sure it's silver solder.
Lead solder will break down and poison the distillate.
That's why people go blind after drinking booze made from stills soldered with lead.)
If you are using a gas or electric stove, you ought to buy a candy thermometer with a six inch metal tube.
I got mine at a restaurant supply store for $4.00.
Most hardware stores carry them.
Alcohol boils at 173 degrees F.
Water boils at 212 degrees F.
If you're distilling alcohol and want pure stuff, the less water coming over, the purer the alcohol.
So you want the temperature just above 173 but considerably below 212.
If you install the thermometer, when alcohol and water starts coming over you can lower the temperature.
When the temperature floats at about 180 degrees you can make a mark near your burner knob.
Next time you use the still you only have to set the knob at the mark and leave it alone.
It will not rise above the temperature you have it set for.
(If all you want to distill is water, or if you just want to drink the alcohol, or don't mind redistilling, you don't really need the thermometer).
To install the thermometer, punch or drill a hole about three inches from the bottom of the can.
Stick the tube as far in as you can and still have room enough to silver-solder or epoxy the thermometer in place.
If silver-solder is used, the thermometer’s dial should be wrapped in a damp rag to prevent damage from the soldering iron's heat.
If you're using a gas stove, the boiler must be a couple of inches above the burner.
Otherwise the fire will go out.
For a stand which would keep the boiler elevated over the flame.
Clyde used a steel cake pan about eight inches in diameter.
I'd want one considerably wider but that size suits Clyde.
Use tin snips to remove the bottom of the pan and to cut large, circular holes around the pan.
Holes large enough to start the tin snips can be made with a drill or a hammer and nail.
The holes in the side of the stand must be large enough to allow plenty of air to get to the gas burner.
To test the stand's air intake, place it on the stove and light the burner.
Put the boiler on the stand and watch the flame.
If it begins to flicker or pop, the vents must be enlarged to increase the air supply.
Now we come to the condenser, which is a round, five gallon paint can, bought used from any paint contractor for a dollar.
This holds the coil and the cooling water.
It doesn't need to be new or even clean, since its contents do not come in contact with whatever is being distilled.
Two holes must be drilled in the condenser can.
The first, about six inches from the top, is the drain.
It is fitted with a two inch copper lube, soldered or epoxied in place.
Attach one end of a two foot length of surgical tubing to this copper tube.
Such tubing, 5/16 of an inch, inside diameter, can be bought from most pharmacies for about 48 cents per foot.
The lower end of this drain tube is held shut with a clothes pin or any convenient clamp.
As the still operates, the water in the condenser will heat up, with the hotter water rising to the top.
The surface water can be almost scalding to the touch, while that a few inches below will be comparatively cool.
Periodically, about a gallon of this hot surface water is drained and replaced with cold water, poured in after the draining.
The second hole is drilled near the bottom of the can at the point where the coil rests on the bottom.
This is the exit hole for the bottom end of the coil.
The coil is made by wrapping ten feel of copper tubing around a can with a smaller diameter than that of the condenser.
Care should be taken in bending the copper tubing lest it collapse.
A good way to prevent this is to plug one end, fill the tubing with fine sand, plug the other end and then bend it to the shape you want.
When the coil is bent, remove the sand.
(Part of the reason I get so depressed and crazy in this work is that some of my readers would leave the sand in and then write and tell me the still worked poorly).
The coil should have a definite downward spiral for maximum efficiency.
Any uphill areas in the coil will trap water.
This will cause the still to spit and sputter as it builds up the necessary pressure to push the trapped water up and over these areas.
Next, place the completed coil in the condenser, with the lower end protruding from the hole about an inch.
Solder or epoxy the copper tubing in place.
The upper end of the coil is left protruding free from the top of the condenser.
This allows it to move as it expands and contracts without the danger of popping the bottom seal and causing a leak.
The boiler and condenser are joined with a removable copper tube about two feet long.
Two two inch long pieces of surgical tubing are used to join the two foot length of copper tubing to the protruding coil and the boiler cap.
The lengths of copper tubing joined by the surgical tubing should be as close as possible so there is little or no surgical tubing exposed to the hot water vapor going from the boiler to the condenser.
Exposed surgical tubing imparts a rubber taste to the distillate.
The surgical tubing leading from the coil to the collecting vessel will not impart a taste since the liquid running through it is cool.
When the distilling process is over, give the boiler cap a twist just to let the outside air pressure get in and equalize the pressure inside the boiler.
If you don't loosen the cap, the greater air pressure outside the boiler will cause it to collapse.
MATERIALS LIST
New five gallon square can with a screw on cap. $2.50;
15 feet of 3/8th inch outside diameter copper tubing. $9.00;
candy thermometer with a five or six inch projection. $4.00;
8 inch diameter steel cake pan. $2.00;
used five gallon paint can. $1.00;
3 feet of 5/16lh inch inside diameter surgical tubing. $1.50.
If joints are to be soldered, silver solder, flux and either a soldering iron or torch are needed.
If epoxy is used, Duro E POX-E Ribbon at $1.89 is best.
Bought from most hardware stores.
It is molded with the fingers.
Remove all grease and dirt from the areas before using epoxy.
Press the epoxy firmly into the open spaces to assure a watertight seal Epoxy breaks down from heat and is not as permanent as silver solder.
If you were a real fanatic about it and kept this still working throughout a 24 hour period you could make at least 36 quarts of at least 100 proof alcohol.
A quart of 100 proof gin or vodka would cost at least $8.00 in the liquor store.
If you sold your alcohol for only $5.00 a quart you could take in $180 per day.

Kurt Saxon thought civilization would have collapsed by now.
He spent the majority of his life collecting knowledge of home based business.
His goal was for all his readers to survive at a more comfortable level than those that were less provident.
He knew the importance of communicating at a level folks could understand.
Most of what he has compiled for our benefit can be easily understood by everybody.
He also includes a subtle sense of humor.
You can find the majority of his life's work here.
Hear him read his stories.
Posts with rewards set to burn only burn the author's portion of the rewards. Curators still get paid.
IF you think this content should be eligible for rewards from the content rewarding pool, please express your discontent in this discord:
Our taps could be our infectors.
It's astounding that people still trust their governments at this point.
Don't think any of those materials are sold by any of those retailers today. That is sad.
Thanks!
Wasted knowledge.
We need to either find replacements available elsewhere, or make them.
https://twitter.com/lee19389/status/1728755171017032128
#hive #posh